|
Boletín de la Sociedad Geológica Mexicana Volumen 74, núm. 2, A150322, 2022 http://dx.doi.org/10.18268/BSGM2022v74n2a150322
|
 |
A small beetle larva preserved in 23-million-year-old Mexican amber: possible first fossil record of an immature variegated mud-loving beetle
Una pequeña larva de escarabajo conservada en ámbar mexicano de 23 millones de años: primer posible registro fósil de un ejemplar inmaduro de escarabajo abigarrado del lodo
Ana Zippel1, Carolin Haug1,2, Joshua Gauweiler1,3, Marie K. Hörnig3, Gideon T. Haug1, Joachim T. Haug1,2,*
1 Biocenter, Ludwig-Maximilians-Universität München (LMU Munich), Großhaderner Str. 2, 82152 Planegg-Martinsried, Germany.
2 GeoBio-Center at Ludwig-Maximilians-Universität München (LMU Munich), Richard-Wagner-Str. 10, 80333 München, Germany.
3 Zoological Institute and Museum, Cytology and Evolutionary Biology, Soldmannstr, University of Greifswald, 23, 17489 Greifswald, Germany.
* Corresponding author: (J.T. Haug) This email address is being protected from spambots. You need JavaScript enabled to view it.
How to cite this article:
Zippel, A., Haug, C., Gauweiler, J., Hörnig, M.K., Haug, G.T., Haug, J.T., 2022, A small beetle larva preserved in 23-million-year-old Mexican amber: possible first fossil record of an immature variegated mud-loving beetle: Boletín de la Sociedad Geológica Mexicana, 74 (2), A150322. http://dx.doi.org/10.18268/BSGM2022v74n2a150322
Manuscript received: February 1, 2022; Corrected manuscript received: March 10, 2022; Manuscript accepted: March 15, 2022
ABSTRACT
Beetles occupy a vast amount of different ecological roles in both terrestrial and freshwater ecosystems. The often very specialised morphology of the adults can frequently be associated with their specific roles. Yet, the ecologically independent larvae of the same species are often unknown; this applies even more so to fossils. Here we report a new fossil beetle larva preserved in Mexican amber. The beetle larva was documented via digital microscopy and X-ray micro-computed tomography (X-ray µCT). The observed features, especially of the trunk, but also the maxillo-labial complex as well as the moulting suture of the head capsule are reminiscent to those of larvae of variegated mud-loving beetles, Heteroceridae. The trunk end is tube-like and protrudes ventro-terminally from abdomen segment 9. While present in other beetle lineages, this morphology is in these lineages, for example, not combined with a simple moulting suture. Some features potentially further supporting an interpretation as a larva of Heteroceridae are not accessible. The interpretation yet remains the most compatible one. Assuming a similar life style to modern larvae of Heteroceridae indicates an original lifestyle associated with running waters, but not within these. Heteroceridae is an ingroup of Byrrhoidea; the fossil record of byrrhoidean larvae is still very scarce. The new fossil hence adds a rare ecological function to the Miocene amber fauna, representing the first fossil record of a larva of Heteroceridae and expanding the fossil record of byrrhoidean larvae.
Keywords: Heteroceridae, Byrrhoidea, Chiapas amber, Miocene, running waters.
RESUMEN
Los escarabajos ocupan una vasta gama de roles ecológicos diferentes, tanto en ecosistemas terrestres como en cuerpos de agua dulce. La morfología de los adultos, frecuentemente especializada, puede a menudo ser asociada con sus roles ecológicos específicos. Sin embargo, las larvas de una misma especie, que usualmente son ecológicamente independientes, son frecuentemente desconocidas; esto aplica con mayor razón al registro fósil. En este trabajo se reporta una larva de escarabajo preservada en ámbar mexicano. La larva de escarabajo fue documentada vía microscopía digital y tomografía microcomputarizada de rayos X (X-ray µCT). Las características observadas, especialmente las del tronco, además del complejo maxilo-labial y la sutura de muda de la cápsula cefálica, son similares a los de larvas de escarabajos abigarrados del lodo, los Heteroceridae. El extremo posterior del tronco es tubular y se protruye en posición ventro-terminal con respecto al segmento 9 del abdomen. Aunque está presente en otros linajes de escarabajos, esta morfología no está combinada, por ejemplo, con una sola estructura de la sutura de la muda. Algunas características que apoyarían aún más la interpretación de esta larva como un Heteroceridae no son accesibles. La interpretación presentada es la más compatible con las evidencias que se puede proporcionar. Asumiendo un modo de vida similar al de las larvas modernas de Heteroceridae, se puede proponer un hábitat asociado a aguas fluviales, aunque no sumergida en ellas. Heteroceridae es un grupo interno de los Byrrhoidea; el registro fósil de las larvas birroideas es aún muy escaso. En virtud de lo anterior, el nuevo fósil implica una rara función ecológica para la fauna del ámbar del Mioceno y representa el primer registro fósil de larvas de Heteroceridae, a la vez que expande el registro de las larvas birroideas.
Palabras clave: Heteroceridae, Byrrhoidea, ámbar de Chiapas, Mioceno, aguas fluviales.
- Introduction
Beetles represent a major component of terrestrial and also freshwater ecosystems. Their ecological roles are manifold and are often highly differentiated between adults and larvae. Not surprisingly, many beetles, adults and larvae, fulfil highly specialised ecological functions in modern ecosystems. We can assume that this was already similar in the past.
Yet, certain assumptions of past ecosystems are more reliable than others. It seems common to conclude from the presence of a specific adult morphology at a specific time slice to the contemporary presence of a specific larval morphology. However, as demonstrated in other lineages of Euarthropoda, such an assumption can be highly misleading (Baranov et al., 2019). Especially in other crustacean groups (Insecta is an ingroup of Crustacea sensu lato, e.g. discussion in Haug and Haug, 2015) it could be demonstrated that larvae and adults have a certain evolutionary independence. This independence can lead to the phenomenon that modern appearing adults are already present, while the specialised larvae evolved much later, or vice versa, with larval morphologies preceding specialised adult morphologies (Haug et al.,2015). Also phylogenetic inferences may be misleading in this aspect, as convergence in closely related lineages may lead to very similar morphologies and developmental patterns (Haug et al., 2013a).
For more reliably reconstructing ecological interactions or even entire ecosystems in the past, it is therefore crucial to indeed look for fossils of larvae, including beetle larvae. Yet, not all types of lifestyles will make it readily likely that such a larva becomes preserved as a fossil. Larvae associated with resin-producing trees have, for example, a higher chance to be preserved in resin. This makes it easy to understand why larvae living directly on such trees are preserved in amber.
Nevertheless, also more unusual larvae such as aquatic ones have been found in various types of ambers. While there have been quite some different interpretations of the occurrence of aquatic organisms in amber (e.g. Wichard et al., 2009) it seems now to be more widely accepted that such organisms indeed became trapped in the amber within their natural habitat (e.g. discussion in Schädel et al., 2019 and references therein). Specialised larvae associated with running waters, but not necessarily living within in them, seem even less likely to be preserved in amber.
We here report a small-sized beetle larva that shows characters of the group Heteroceridae. Extant larvae of this group are known to live in sandy shores of, for example, rivers. We discuss indications of this new find.
- Material and Methods
Material: In the centre of this study is a single specimen preserved in early Miocene (Aquitanian) Chiapas amber, Mexico (Serrano-Sánchez et al. 2015). The specimen was legally purchased from the amber trader gemgazer.com. The specimen is now deposited in the Palaeo-Evo-Devo Research Group Collection of Arthropods, Ludwig-Maximilians-Universität München, Germany, under repository number PED 0895.
Microscopic documentation: The specimen was initially documented on a Keyence VHX-6000 digital microscope, following standard procedures (e.g., Haug et al., 2013b; Haug and Haug, 2019). This includes composite imaging in x-, y- and z-axis (Haug et al., 2011) as well as HDR (Haug et al., 2013c).
X-ray micro-computed tomography (µCT): The specimen was further documented with a XRadia MicroXCT-200 (Carl Zeiss Microscopy GmbH, Jena, Germany), which is equipped with switchable scintillator-lens units. 1600 projections were recorded with following settings: 10x objective; source settings 40 kV, 8 W; 6s exposure time; binning 2. System-based calculated pixel size is 1.43 µm, 1005 x 1005 px. Projections were further processed with binning 1 using the XMReconstructor software (Carl Zeiss Microscopy GmbH, Jena, Germany), resulting in image stacks (TIFF format). Volume renderings based on the µCT data were processed using OSIRIX.
- Description of the specimen
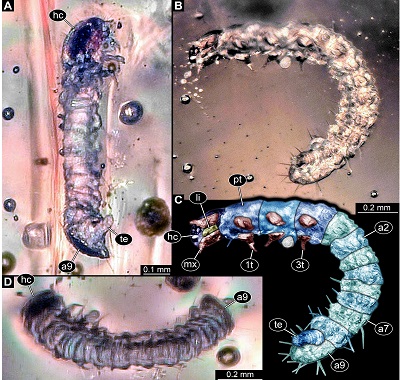
General: Small-sized beetle larva with total body length of ~1.61 mm. Body elongate cylindrical, only slightly dorso-ventrally flattened (Figure 1). Organised in anterior head and elongate trunk (Figure 2). Trunk further differentiated into anterior (thorax) and posterior region (abdomen) (Figure 2). Head slightly flattened dorso-ventrally, prognathous (mouth parts facing forwards) (Figures 2C to 2I). Antennae discernible, mouth parts prominent. Anterior trunk with three segments (pro-, meso-, metathorax). Thorax with one pair of appendages (legs) on each segment (Figure 1). Posterior trunk, abdomen, with ten discernible units, nine segments and terminal end (possibly compound structure; Figure 1). Abdomen segment 9 with posteriorly drawn-out tergite (Figure 3). Terminal end elongate, orientated postero-ventrally, not discernible in dorsal view (Figures 1A–1C, 3B–3E).
 |
|
Figure 1.New beetle larva from Mexican amber, PED 0895, light microscopy. A. Oblique ventral view. B. Oblique lateral view. C. Colour-marked version of B. D. Oblique dorsal view. Abbreviations: 1t–3t= trunk appendages (“legs”) 1–3; a2–a9 = abdomen segments 2–9; hc = head capsule; li = labium; mx = maxilla; pt = prothorax; te = trunk end. |
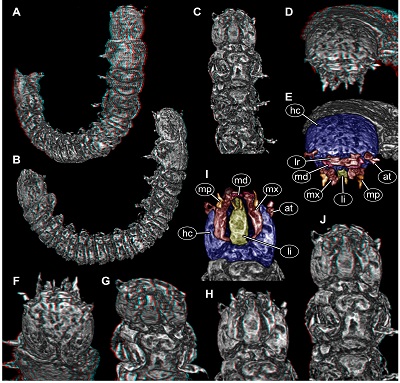 |
| Figure 2. New beetle larva from Mexican amber, PED 0895, volume renderings based on µCT. A–D. Red-cyan stereo anaglyphs. A, B. Overviews. A. Oblique dorsal view. B. Oblique lateral view. C. Anterior body, ventral view. D. Antero-dorsal view on head. E. Colour-marked version of D. F–H. Red-cyan stereo anaglyphs of head region. F. In dorsal view. G. In antero-ventral view. H. In ventral view. I. Colour-marked version of H. J. Red-cyan stereo anaglyph of anterior body region in postero-ventral view. Please use red-cyan glasses to view stereo anaglyphs. Images not to scale. Abbreviations: at = antenna; hc = head capsule; li = labium; lr = labrum; md = mandible; mp = maxillary palp; mx = maxilla. |
Head: Almost circular in dorsal view, flattened in lateral view (Figures 2A, 2B and 3A), as long as wide (~0.13 mm), dorsal surface with Y-shaped suture (ecdysis lines) (Figure 2F). No stemmata discernible. Antenna (appendage of post-ocular segment 1) short, with two discernible elements (Figure 2E and 2I), ~0.03 mm long. Second element with a process almost as long as main part of element (Figure 2E).
Mouth parts discernible (Figure 2). Labrum (derivative of ocular segment) relatively large, sub-rectangular in antero-dorsal view (Figure 2E). No spines or setae discernible.
Mandibles (appendages of post-ocular segment 3) partially accessible (Figures 2E, 2I), yet without details.
Maxillae (appendages of post-ocular segment 4) elongate, subrectangular in ventral view (Figure 2I), longer than wide, 2.5x (~0.15 mm long). Proximally slightly wider due to medially drawn-out part, distally with palp (~0.03 mm long), presumably with multiple elements.
Labium (conjoined appendages of post-ocular segment 5) elongate, triangular in ventral view (Figure 2I), longer than wide, 3x (~0.09 mm long), distally with a pair of palps (Figure 2I).
Thorax: Prothorax sub-trapezoidal in dorsal view, anterior rim slightly wider than posterior rim, lateral sides rounded (Figure 2A), as long as wide at the posterior rim (~0.19 mm). Tergite of prothorax (pronotum) with prominent median longitudinal line, lateral sides prolonged and drawn-down ventrally. Single seta discernible (Figures 1B,1C).
Mesothorax sub-rectangular in dorsal view, slightly shorter than prothorax (~0.18 mm), as wide as the posterior rim of prothorax (Figure 2A).
Metathorax sub-similar to mesothorax, shorter than prothorax, (~0.16 mm), as wide as the posterior rim of prothorax (Figure 2A). Tergites of meso- and metathorax with transverse lines.
Legs discernible on all three segments, ~0.11 mm long. Yet, details, as e.g. exact number of elements not accessible (Figures 1A–1C, 2C and 2J).
Abdomen: Abdomen segments 1–8 subsimilar (Figures 1B to 1D), wider than long, measuring between 0.06–0.11 mm in length (with segment 5 being shortest and segments 1 and 8 longest) and between 0.19–0.22 mm in width (with segment 8 being widest). Lateral sides of tergites of segments 1–8 prolonged and drawn-down ventrally (Figures 2A and 2B).
Abdomen segment 9 semi-ovoid in ventral and dorsal view, longer than wide, 1.4x (~0.22 mm long), tergite drawn-out posteriorly, longer than ventral region of segment 3.1x (ventral region ~0.07 mm long). Few setae discernible on abdomen segments 5–9, no exact arrangement (chaetotaxy) reconstructible.
Terminal end longer than wide, 2.3x (~0.16 mm long) hidden by posteriorly drawn-out part of tergite of segment 9 in dorsal view. Ventrally wider, tapering distally (Figure 3B and 3C). Distal part ending in two symmetrical, short processes (Figures 3D and 3E).
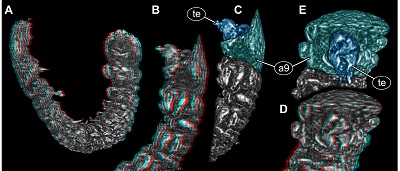 |
| Figure 3.New beetle larva from Mexican amber, PED 0895, volume renderings based on µCT, continued. A, B. Red-cyan stereo anaglyphs. A. Overview in oblique lateral view. B. Posterior trunk region in lateral view. C. Colour-marked version of B. D. Red-cyan stereo anaglyph of posterior trunk region in posterior view. E. Colour-marked version of D. Please use red-cyan glasses to view stereo anaglyphs. Images not to scale. Abbreviations: a9 = abdomen segment 9; te = trunk end. |
- Discussion
Beetle larvae preserved in amber: Klausnitzer (2003) assumed that beetle larvae are rare in (Baltic) amber and difficult to interpret. This evaluation seems to be based on the fact that beetle larvae are only rarely reported as fossils, leaving the impression that they are rare. Yet, fossil beetle larvae may in fact be much more common, but only rarely investigated and published.
For other groups of Holometabola it could be well demonstrated that the seeming rareness of larvae in amber is simply an effect of low sampling and especially non-specific sampling. Aimed search for specific larvae revealed that some of the presumed rare forms are more common than originally expected (Neuroptera: e.g., Pérez-de la Fuente et al., 2020; Haug et al.,2020a, 2021a; Diptera: e.g., Baranov et al., 2019, 2020, 2021).
Why is it worth to search for fossil larvae at all? Numerous reasons have been given why the focus on adults is in fact not based on logical reasons but a convention, and why larvae add to research in various ways (see, for example, discussions in Haug and Haug 2016, 2017 and references therein). Especially in holometabolan groups the often strongly differing larvae with their usually also strongly differing ecology can be taken as prime examples. The relative evolutionary independence of adults and larvae (Scholtz, 2005) is so strongly expressed in beetles, butterflies and their relatives that ignoring their immatures, especially in the fossil record, is not comprehensible.
The practical challenges of dealing with beetle larvae in amber are another part of the story. Most features that a determination key for extant beetle larvae requires are not accessible in fossils. Klausnitzer (2003) suggested that only larvae with very pronounced features can be properly determined. It appears indeed easier to recognise very special larvae such as, for example, the larvae of texas beetles (Brachypsectridae) with their specific head and a trunk with very unusual and complex lateral processes (Zhao et al., 2020; Haug et al., 2021b). Also larvae of wriggling beetles (Gyrinidae; Zhao et al.,2019; Gustafson et al., 2020) or certain larvae of false flower beetles (Scraptiidae; Haug and Haug, 2019; Zippel et al., 2022) with enlarged trunk ends can be easily identified as such based on prominent structures. Another problem occurs with larvae that are very special in appearance, but unknown in the modern fauna (Haug et al.,2020b). This lack of knowledge does not mean that such larvae are not present in the modern fauna, as the immature stages of the majority of modern beetles (and many other holometabolans) is still unknown.
Especially in very small beetle larvae it can be challenging to provide any guess of their identity as even fewer details are often accessible. Access with optical methods is limited in amber due to the optical properties of the amber, usually meaning that the lower border of resolution is way above the Abbe limit. This phenomenon is mainly caused by irregularities of the amber, resulting in refractive artefacts. Also modern µCT imaging does not necessarily improve this specific aspect, since depending on the preservation of the specimen, the x-ray absorption of inclusion and surrounding amber can be very similar (resulting in low contrast), and metallic particles can cause strong artefacts. This is especially problematic with small specimens in amber, however, µCT is still able to improve access to concealed and verlumt details. Luckily, the specimen at hand has some features which can be quite informative.
Comparison of the fossil to extant beetle larvae: The posterior end of the fossil larva is quite prominent, with the trunk end being cone-like, protruding from abdomen segment 9 postero-ventrally. Such an arrangement is well known in a number of beetle larvae: For example, in at least some larvae of the groups Lymexilidae (Wheeler, 1991, Figure 34.498–34.500 p. 447), Cleridae (Foster and Lawrence, 1991, Figure 34.508 p. 451), Nitidulidae (Lawrence, 1991a, Figure 34.514a p. 458), Rhizophagidae (Lawrence, 1991b, Figure 34.523a p. 461), Cryptophagidae (Lawrence, 1991c, Figure 34.539 p. 470), Languriidae (Lawrence, 1991d, Figure 34.546 p. 472), Erotylidae (Lawrence 1991e, Figure 34.548 p. 474), Melandryidae (Lawrence, 1991f, 34.643a p. 507) and Elateridae (Becker, 1991, Figures 34.437a, 34.439, 34.440 all on p. 415) we find an overall similar arrangement. Yet, in all these cases abdomen segment 9 bears prominent processes or urogomphi, which are clearly not present in the larva at hand. There is also no indication that such structures were originally present in the fossil, but broke off.
The similarity to certain larvae of Elateridae is even higher concerning the shape of abdomen segment 9, at least in dorsal view. (e.g. Becker, 1991, Figure 34.436 p. 415, Figures 34.450, 34.451 both on p. 417). Yet, these larvae differ in other aspects. In lateral or ventral view, abdomen segment 9 differs from that of the new larva in lacking a distinct ventral region. Also larvae of Elateridae have a special arrangement of the moulting sutures on the head capsule, with a vase-shaped middle part (e.g. Becker, 1991, Figure 34.437b p. 415). In the fossil, the sutures form a simple Y.
A more distantly similar morphology of the trunk end is also known in few larvae of Tenebrionidae (Lawrence and Spilman, 1991, Figure 34.672a, d p. 525). However, in these larvae the trunk end appears quite differentiated, while in the fossil it appears rather simple, soft and tubular. Such a pygopod-like morphology of a trunk end can also be seen in some larvae of Chrysomelidae (Lawson, 1991, e.g. Figure 34.835 p. 582), yet these differ significantly in overall appearance compared to the state in the new fossil.
Distantly similar trunk ends are also present in larvae of Byrrhoidae (Lawrence, 1991f, Figure 34.387–389 p. 385), Ptilodactylidae (Lawrence, 1991g, Figure 34.403 p. 392) and Artematopidae (Cooper, 1991, Figure 34.433a p. 408). Yet, the largest similarity concerning the shape of the trunk end is in fact seen in larvae of Heteroceridae. Here the trunk end is distinctly pygopod-like (e.g. Vanin et al., 1995, Figures 11, 12 both on p. 107).
Also other characters are compatible with an interpretation of the fossil larva as representative of the group Heteroceridae. The moulting sutures of the head capsule are simple and Y-shaped in extant larvae, yet, unlike in the fossil, the middle branch is much shorter. The antennae in larvae of Heteroceridae are very small (Lawrence 1991h p. 402; Vanin et al., 1995, Figure 15 p. 108, Figure 19 p. 109) and are distally bifid (Vanin et al., 1995, Figure 15 p. 108), which seems also to be the case in the new fossil (Figures 3D and 3E). Also the prominent maxillo-labial complex is very similar in the modern larvae and the fossil with short maxillary palps, very broad proximal (posterior) end of the labium and very short labial palps.
There are certain differences of the fossil larvae, as for example the rather wide abdomen segment 9, which is about as wide as the head capsule. In the known larvae of Heteroceridae, abdomen segment 9 is always narrower than the head capsule, but in fact only very few larvae of Heteroceridae are known (examples in Figure 4). Moreover, all known larvae seem to be late stage larvae, while the fossil, based on its small size, is likely an early stage larva. The phenomenon that mostly late stage larvae are known in the modern fauna, but only early stage larvae are known as fossils, has been noticed in at least one other beetle group (Scraptiidae), and the resulting problems have been discussed (Haug and Haug, 2019; Zippel et al., 2022).
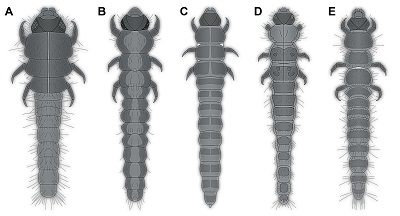 |
| Figure 4. Examples of extant larvae of Heteroceridae, modified from the literature. A–D. From Pierre (1945). A. His figure 1. B. His figure 18. C. His figure 19. D. His figure 20. E. From Vanin et al., (1995), their figure 10. |
Certain morphological aspects of the fossil larva, which could further support an interpretation as a larva of Heterocerdiae, such as scattered stemmata, details of the mandibles or specialised spiracles (Lawrence, 1991h, pp. 402–403) are not accessible in the fossil.
We can therefore only conclude that an interpretation of the larva as representative of Heteroceridae is compatible with the observable details and represents the currently best interpretation. Details possibly further corroborating this interpretation are not available.
Ecology of modern-day larvae of Heteroceridae and fossil record of the group: Modern larvae of Heteroceridae live on the sandy shores of water bodies (rivers, ponds, but also bodies of brackish or even salty water) and dig tunnels through the wet sand. They seem to ingest substrate, extracting algae and other micro-organisms as food (e.g, see discussion in Vanin et al., 1995 p. 104; Sazhnev, 2018, 2020).
So far, very few fossils of Heteroceridae have been reported, largely restricted to adult specimens (Ponomarenko, 1986; Prokin and Ren, 2011; Li et al., 2020). The only other record is a supposed trace fossil of tunnels (Clark and Ratcliffe 1989). Yet, as could be shown, very similar traces can be left also by other organisms, making a clear identification challenging (Metz, 1990).
What does this mean for the new larva? We know that the earliest representatives of the group were already present about 100 million years ago (Li et al., 2020), hence it is reasonable to have fossils in 23-million-year-old amber from early Miocene (Serrano-Sánchez et al., 2015). Also the group is represented in the modern fauna of Mexico (e.g. Skalicky, 2018, 2021). Although it has been speculated that aquatic organisms preserved in amber can only be explained by very unusual circumstances (e.g. Wichard et al., 2009) it indeed appears that in certain amber types aquatic organisms are preserved in situ (e.g. Schmidt et al., 2018; Xing et al., 2018; Yang et al., 2019; Zhao et al.,2019; Gustafson et al., 2020; Wang et al., 2020; Schädel et al., 2020, 2021a, 2021b; Haug et al., 2021c; Baranov et al., 2022). Also Mexican amber is well known to preserve aquatic organisms (Huys et al., 2016; Serrano-Sánchez et al., 2016; Du et al., 2019; Matzke-Karasz et al., 2019). Hence the preservation of animals living directly associated to water bodies, such as larvae of Heteroceridae, seems well likely. In summary, an interpretation of the new larva as a representative of Heteroceridae, with a similar lifestyle to modern forms, is not contradicted by ecological and preservational circumstances.
Larvae in Chiapas amber: As pointed out, larvae preserved in amber are less often reported than adults. So far only few larvae have been explicitly reported from Chiapas amber (e.g. Poinar and Poinar, 2005; Serrano-Sánchez et al., 2016; Haug et al., 2021d). Yet, apparently such individuals can well be preserved.
It is well possible to use larval forms for comparisons of diversity, if sufficient material can be gathered for different time slices (e.g. Haug et al., 2020a, 2021a). While Mexican amber is not the only source of amber from the Miocene, it is especially important, as it preserves components of the aquatic fauna and animals associated with water (e.g. Solórzano-Kraemer, 2010, Serrano-Sánchez et al., 2015), as presumed for the new larva. Such components appear to be largely absent in Miocene Dominican amber. Hence, Mexican amber is a good source of information for making our view on the Miocene fauna more complete. It will also be important to continue to report more larvae, such as the fossil here, to add ecological roles so far not directly recognised.
Contributions of authors
(1) conceptualization (A.Z.; C.H.;J.G.; M.K.H.; G.T.H.;J.T.H.), (2) analysis or data acquisition (A.Z.; C.H.;J.G.; M.K.H.), (3) methodologic/technical development (A.Z.; C.H.;J.G.; G.T.H.;J.T.H.), (4) writing of the original manuscript, (A.Z.; C.H.;J.G.; M.K.H.; G.T.H.;J.T.H.), (5) writing of the corrected and edited manuscript( A.Z.; C.H.;J.G.; M.K.H.; G.T.H.;J.T.H.), (6) graphic design (A.Z.; C.H.;J.G.; G.T.H.;J.T.H.),(7) fieldwork (A.Z.; C.H.;J.G.; M.K.H.; G.T.H.;J.T.H.) (8) interpretation (A.Z.; C.H.;J.G.; M.K.H.; G.T.H.;J.T.H.).
Financing
JTH is supported by the Volkswagen Foundation (Lichtenberg professorship) and by the German Research Foundation (DFG Ha 6300/6-1). Micro-computed tomography was performed at the Imaging Center of the Department of Biology, University of Greifswald (DFG INST 292/119-1 FUGG; DFG INST 292/120-1 FUGG).
Acknowledgements
Our thanks go to J. Matthias Starck, Munich, for long-time support. We are grateful to all people providing low-cost, open-access or open-source software. This is LEON publication #39.
Conflicts of interest
The authors declare that there is no conflict of interest with other authors, institutions or other third parties in relation to the content of this article.
References
Baranov, V.A., Schädel, M., Haug, J.T., 2019, Fly palaeo-evo-devo: immature stages of bibionomorphan dipterans in Baltic and Bitterfeld amber: PeerJ 7, e7843. https://doi.org/10.7717/peerj.7843
Baranov, V.A., Wang, Y., Gašparič, R., Wedmann, S., Haug, J.T., 2020, Eco-morphological diversity of larvae of soldier flies and their closest relatives in deep time: PeerJ 8, e10356. https://doi.org/10.7717/peerj.10356
Baranov, V.A., Engel, M. S., Hammel, J., Hörnig, M.K., van de Kamp, T., Zuber, M., Haug, J.T., 2021, Synchrotron-radiation computed tomography uncovers ecosystem functions of fly larvae in an Eocene forest: Palaeontologia Electronica, 24(1), a07. https://doi.org/10.26879/1129
Baranov, V., Haug, C., Fowler, M., Kaulfuss, U., Müller, P., Haug, J.T., 2022, Summary of the fossil record of megalopteran and megalopteran-like larvae, with a report of new specimens: Bulletin of Geosciences, 97, 89–108. https://doi.org/10.3140/bull.geosci.1840
Becker, E.C., 1991, Elateridae (Elateroidea) (including Dicronychidae, Lissomidae), in Stehr, F.W. (ed.), Immature Insects, 2: Dubuque, Iowa (Kendall), 410–417.
Clark, G. R., Ratcliffe, B. C., 1989, Observations on the tunnel morphology of Heterocerus brunneus Melsheimer (Coleoptera: Heteroceridae) and its paleoecological significance: Journal of Paleontology 63, 228–232. https://doi.org/10.1017/s0022336000019259
Cooper, K. W., 1991, Artematopidae (Elateroidea) (= Eurypogonidae). In Stehr, F.W. (ed.), Immature Insects, 2,: Dubuque, Iowa (Kendall), 407–409.
Du, B.J., Chen, R., Li, X.Z., Tao, W.T., Bu, W.J., Xiao, J.H., Huang, D.W., 2019, The first amber caridean shrimp from Mexico reveals the ancient adaptation of the Palaemon to the mangrove estuary environment: Scientific Reports 9(1), 14782. https://doi.org/10.1038/s41598-019-51218-5
Foster, D.E., Lawrence, J.F., 1991, Cleridae (Cleroidea) (including Corynetidae, Korynetidae), in Stehr, F. W. (ed.), Immature Insects, 2,: Dubuque, Iowa (Kendall), 450–452.
Gustafson, G.T., Michat, M.C., Balke, M., 2020, Burmese amber reveals a new stem lineage of whirligig beetle (Coleoptera: Gyrinidae) based on the larval stage: Zoological Journal of the Linnean Society 189, 1232–1248. https://doi.org/10.1093/zoolinnean/zlz161
Haug, C., Haug, J.T., 2016, Developmental Paleontology and Paleo-Evo-Devo. In Kliman, R.M. (ed.), Encyclopedia of Evolutionary Biology, vol. 1. Oxford, Academic Press, 420–429.
Haug, C., Haug, J.T., 2017, Methods and Practices in Paleo-Evo-Devo, in Nuño de la Rosa, L., Müller, G.B., (eds.), Evolutionary Developmental Biology: Springer International Publishing, 1–14. https://doi.org/10.1007/978-3-319-33038-9_41-1
Haug, C., Shannon, K.R., Nyborg, T., Vega, F.J. 2013c, Isolated mantis shrimp dactyli from the Pliocene of North Carolina and their bearing on the history of Stomatopoda: Bolétin de la Sociedad Geológica Mexicana 65, 273–284. https://doi.org/10.18268/bsgm2013v65n2a9
Haug, C., Haug, G.T., Baranov, V.A., Solórzano-Kraemer, M.M., Haug, J.T., 2021d, An owlfly larva preserved in Mexican amber and the Miocene record of lacewing larvae: Boletín de la Sociedad Geológica Mexicana, 73(3), A271220. https://doi.org/10.18268/bsgm2021v73n3a271220
Haug, G.T., Haug, C., Pazinato, P. G., Braig, F., Perrichot, V., Gröhn, C., Müller, P., Haug, J.T., 2020a, The decline of silky lacewings and morphological diversity of long-nosed antlion larvae through time: Palaeontologia Electronica 23(2), a39. https://doi.org/10.26879/1029
Haug, G.T., Haug, C., van der Wal, S., Müller, P., Haug, J.T., 2021a, Split-footed lacewings declined over time: indications from the morphological diversity of their antlion-like larvae: PalZ, 96(1), 29-50. https://doi.org/10.1007/s12542-021-00550-1
Haug, J.T., Haug, C., 2015, “Crustacea”: Comparative aspects of larval development, in Wanninger, A., (ed.), Evolutionary Developmental Biology of Invertebrates 4: Ecdysozoa II: Crustacea. Springer, Wien, 1–37. https://doi.org/10.1007/978-3-7091-1853-5_1
Haug, J.T., Haug, C., 2019, Beetle larvae with unusually large terminal ends and a fossil that beats them all (Scraptiidae, Coleoptera): PeerJ, 7, e7871. https://doi.org/10.7717/peerj.7871
Haug, J.T., Haug, C., Kutschera, V., Mayer, G., Maas, A., Liebau, S., Castellani, C., Wolfram, U., Clarkson, E.N.K., Waloszek, D., 2011, Autofluorescence imaging, an excellent tool for comparative morphology: Journal of Microscopy, 244, 259–272. https://doi.org/10.1111/j.1365-2818.2011.03534.x
Haug, J.T., Audo, D., Charbonnier, S., Haug, C., 2013a, Diversity of developmental patterns in achelate lobsters-today and in the Mesozoic: Development Genes and Evolution, 223, 363–373. https://doi.org/10.1007/s00427-013-0452-x
Haug, J.T., Müller, C.H.G., Sombke, A., 2013b, A centipede nymph in Baltic amber and a new approach to document amber fossils: Organisms Diversity and Evolution, 13, 425–432. https://doi.org/10.1007/s13127-013-0129-3
Haug, J.T., Martin, J.W., Haug, C., 2015, A 150-million-year-old crab larva and its implications for the early rise of brachyuran crabs: Nature Communications, 6(1), 6417. https://doi.org/10.1038/ncomms7417
Haug, J.T., Schädel, M., Baranov, V.A., Haug, C., 2020b, An unusual 100-million-year old holometabolan larva with a piercing mouth cone: PeerJ, 8, e8661. https://doi.org/10.7717/peerj.8661
Haug, J.T., Müller, P., Haug, C. 2021c. Fossil dragonfly-type larva with lateral abdominal protrusions and implications on the early evolution of Pterygota: iScience, 24(10), 103162. https://doi.org/10.1016/j.isci.2021.103162
Haug, J.T., Zippel, A., Haug, G.T., Hoffeins, C., Hoffeins, H.W., Hammel, J.U., Baranov, V.A., Haug, C., 2021b, Texas beetle larvae (Brachypsectridae)–the last 100 million years reviewed: Palaeodiversity, 14, 161–183. https://doi.org/10.18476/pale.v14.a8
Huys, R., Suárez-Morales, E., Serrano-Sánchez, M.L., Centeno-García, E., Vega, F.J., 2016, Early Miocene amber inclusions from Mexico reveal antiquity of mangrove-associated copepods: Scientific Reports, 6, 34872. https://doi.org/10.1038/srep34872
Klausnitzer, B., 2003, Käferlarven (Insecta: Coleoptera) in Baltischem Bernstein-Möglichkeiten und Grenzen der Bestimmung: Entomologische Abhandlungen 61, 103–108.
Lawrence, J.F., 1991a, Nitidulidae (Cucujoidea) (including Brachypteridae, Cateretidae, Cybocephalidae, Smicripidae), in Stehr, F.W. (ed.), Immature Insects, 2: Dubuque, Iowa (Kendall), 456–460.
Lawrence, J.F., 1991b, Rhizophagidae (Cucujoidea) (including Monotomidae), in Stehr, F.W. (ed.), Immature Insects, 2: Dubuque, Iowa (Kendall), 460–462.
Lawrence, J.F., 1991c, Cryptophagidae (Cucujoidea) (including Catopochrotidae, Hypocopridae), in Stehr, F.W. (ed.), Immature Insects, 2: Dubuque, Iowa (Kendall), 469–471.
Lawrence, J.F., 1991d, Languriidae (Cucujoidea) (including Cryptophilidae), in Stehr, F.W. (ed.), Immature Insects, 2: Dubuque, Iowa (Kendall), 471–473.
Lawrence, J.F., 1991e, Erotylidae (Cucujoidea) (including Dacnidae), in Stehr, F.W. (ed.), Immature Insects, 2: Dubuque, Iowa (Kendall), 473–475.
Lawrence, J.F., 1991f, Byrrhidae (Byrrhoidea) (including Syncalyptidae), in Stehr, F.W. (ed.), Immature Insects, 2: Dubuque, Iowa (Kendall), 384–386.
Lawrence, J.F.,1991g, Ptilodactylidae (Dryopoidea), in Stehr, F.W. (ed.), Immature Insects, 2: Dubuque, Iowa (Kendall), 391–394.
Lawrence, J.F., 1991h, Heteroceridae (Dryopoidea), in Stehr, F.W. (ed.), Immature Insects, 2: Dubuque, Iowa (Kendall), 402–404.
Lawrence, J.F., Spilman, T.J., 1991, Tenebrionidae (Tenebrioniodea) (including Alleculidae, Cossyphodidae, Lagriidae, Nilionidae, Rhysopaussidae, Tentyriidae), in Stehr, F.W. (ed.), Immature Insects, 2: Dubuque, Iowa (Kendall), 520–528.
Lawson, F.A., 1991, Chrysomelidae (Chrysomeloidea) (= Cassididae, Cryptocephalidae, Megalopodidae, Sagiridae, etc.), in Stehr, F.W. (ed.), Immature Insects, 2: Dubuque, Iowa (Kendall), 568–585.
Li, Y., Tihelka, E., Huang, D., Cai C., 2020, Specialized variegated mud-loving beetles from mid-Cretaceous Burmese amber (Coleoptera: Heteroceridae): Palaeoentomology 3, 059–067. https://doi.org/10.11646/palaeoentomology.3.1.9
Matzke-Karasz, R., Serrano-Sánchez, M.D.L., Pérez, L., Keyser, D., Pipík, R.,Vega, F.J., 2019, Abundant assemblage of Ostracoda (Crustacea) in Mexican Miocene amber sheds light on the evolution of the brackish-water tribe Thalassocypridini: Historical Biology 31, 65–101. https://doi.org/10.1080/08912963.2017.1340471
Metz, R., 1990, Tunnels formed by mole crickets (Orthoptera: Gryllotalpidae): paleoecological implications: Ichnos 1, 139–141.
Pérez de la Fuente, R., Engel, M.S., Delclòs, X., Peñalver, E., 2020, Straight-jawed lacewing larvae (Neuroptera) from Lower Cretaceous Spanish amber, with an account on the known amber diversity of neuropterid immatures: Cretaceous Research, 106, 104200. https://doi.org/10.1016/j.cretres.2019.104200
Pierre, F., 1945, La larve d’Heterocerus aragonicus Kiesw. et son milieu biologique: Revue Française d’Entomologie Générale 12, 166–174.
Poinar Jr, G., Poinar, R., 2005, Fossil evidence of insect pathogens: Journal of Invertebrate Pathology, 89, 243–250. https://doi.org/10.1016/j.jip.2005.05.007
Ponomarenko, A.G., 1986, Coleoptera. Scarabaeida (=Coleoptera), in Nasekomye v rannemelovykh ekosistemakh Zapadnoi Mongolii (Insects in the Early Cretaceous Ecosystems of Western Mongolia), Moscow: Nauka, 84–105.
Prokin, A.A., Ren, D., 2011, New species of variegated mud-loving beetles (Coleoptera: Heteroceridae) from mesozoic deposits of China: Paleontological Journal, 45, 284–286. https://doi.org/10.1134/s003103011102016x
Sazhnev, A.S., 2018, On the position of Heteroceridae (Insecta: Coleoptera) in food webs in riparian communities: Ecosystem Transformation, 1, 49–56. https://doi.org/10.23859/estr-180121
Sazhnev, A.S., 2020, Beetles of the family Heteroceridae (Insecta: Coleoptera) in extreme environments: Ecosystem Transformation 3(2), 84–93. https://doi.org/10.23859/estr-200323a
Schädel, M., Perrichot, V., Haug, J.T., 2019, Exceptionally preserved cryptoniscium larvae - morphological details of rare isopod crustaceans from French Cretaceous Vendean amber: Palaeontologia Electronica, 22(3), 71. https://doi.org/10.26879/977
Schädel, M., Müller, P., Haug, J.T., 2020, Two remarkable fossil insect larvae from Burmese amber suggest the presence of a terminal filum in the direct stem lineage of dragonflies and damselflies (Odonata): Rivista Italiana di Paleontologia e Stratigrafia, 126, 13–35. https://doi.org/10.13130/2039-4942/12720
Schädel, M., Hörnig, M.K., Hyžný, M., Haug, J.T., 2021a, Mass occurrence of small isopodan crustaceans in 100-million-year-old amber: an extraordinary view on behaviour of extinct organisms: PalZ, 95, 429–445. https://doi.org/10.1007/s12542-021-00564-9
Schädel, M., Hyžný, M., Haug, J.T., 2021b, Ontogenetic development captured in amber-the first record of aquatic representatives of Isopoda in Cretaceous amber from Myanmar: Nauplius, 29, e2021003. https://doi.org/10.1590/2358-2936e2021003
Schmidt, A.R., Grabow, D., Beimforde, C., Perrichot, V., Rikkinen, J., Saint Martin, S, Thiel, V., Seyfullah, L.J., 2018, Marine microorganisms as amber inclusions: insights from coastal forests of New Caledonia: Fossil Record, 21, 213–221. https://doi.org/10.5194/fr-21-213-2018
Scholtz, G., 2005, Homology and ontogeny: pattern and process in comparative developmental biology: Theory in Biosciences, 124, 121–143. https://doi.org/10.1016/j.thbio.2005.09.002
Serrano-Sánchez, M.L., Hegna, T.A., Schaaf, P., Pérez, L., Centeno-García, E., Vega, F.J., 2015, The aquatic and semiaquatic biota in Miocene amber from the Campo LA Granja mine (Chiapas, Mexico): Paleoenvironmental implications: Journal of South American Earth Sciences, 62, 243–256. https://doi.org/10.1016/j.jsames.2015.06.007
Serrano-Sánchez, M.L., Nagler, C., Haug, C., Haug, J.T., Centeno-García, E., Vega, F.J., 2016, The first fossil record of larval stages of parasitic isopods: cryptoniscus larvae preserved in Miocene amber: Neues Jahrbuch für Geologie und Paläontologie, Abhandlungen 279(1), 97–106. https://doi.org/10.1127/njgpa/2016/0543
Solórzano-Kraemer, M.M., 2010, Mexican amber, in Penney, D. (ed.), Biodiversity of fossils in amber from the major world deposits: Manchester, Siri Scientific Press, 42–56.
Skalicky, S., 2018, Heterocerus evzeni nov. sp. from Mexico (Coleoptera, Heteroceridae): Linzer Biologische Beiträge 50(2), 1657–1660. http://doi.org/10.5281/zenodo.5275203
Skalicky, S., 2021, Tropicus mareki sp. nov. from Mexico (Insecta: Coleoptera: Heteroceridae): Studies and Reports Taxonomical Series, 17(1), 133–137.
Vanin, S.A., Costa, C., Gianuca, N.M., 1995, Larvae of Neotropical Coleoptera XXI: Description of immatures and Ecology of Efflagitatus freudei Pacheco, 1973 (Dryopoidea, Heteroceridae): Iheringia, Série Zoologia 78, 99–112.
Wang, H., Schädel, M., Sames, B., Horne, D.J., 2020, New record of podocopid ostracods from Cretaceous amber: PeerJ, 8, e10134. https://doi.org/10.7717/peerj.10134
Wheeler, Q., 1991, Lymexylidae (Lymexyloidea), in Stehr, F.W. (ed.), Immature Insects, 2, Dubuque, Iowa (Kendall), 446–447.
Wichard, W., Gröhn, C., Seredszus, F., 2009, Aquatic Insects in Baltic Amber: Wasserinsekten im Baltischen Bernstein:Germany, Verlag Kessel, Remagen-Oberwinter, 336 p.
Xing, L., Sames, B., McKellar, R.C., Xi, D., Bai, M., Wan, X., 2018, A gigantic marine ostracod (Crustacea: Myodocopa) trapped in mid-Cretaceous Burmese amber: Scientific Reports, 8, 1365. https://doi.org/10.1038/s41598-018-19877-y
Yang, Q., Chen, Z.Y., Jia, F.L., 2019, Ambarticus myanmaricus gen. et sp. nov., the first diving beetle from mid-Cretaceous amber of northern Myanmar (Coleoptera, Dytiscidae, Dytiscinae): Cretaceous Research, 102, 1–6. https://doi.org/10.1016/j.cretres.2019.05.005
Zhao, X., Zhao, X., Jarzembowski, E.A., Wang, B., 2019, The first whirligig beetle larva from mid-Cretaceous Burmese amber (Coleoptera: Adephaga: Gyrinidae): Cretaceous Research, 99, 41–45. https://doi.org/10.1016/j.cretres.2019.02.015
Zhao, X., Zhao, X., Jarzembowski, E., Tian, Y., Chen, L., 2020, The first record of brachypsectrid larva from mid-Cretaceous Burmese amber (Coleoptera: Polyphaga): Cretaceous Research 113, 104493. https://doi.org/10.1016/j.cretres.2020.104493
Zippel, A., Haug, C., Hoffeins, C., Hoffeins, H.W., Haug, J.T., 2022, Expanding the record of larvae of false flower beetles with prominent terminal ends: Rivista Italiana di Paleontologia e Stratigrafia, 128, 81–104. https://doi.org/10.54103/2039-4942/17084

