|
Boletín de la Sociedad Geológica Mexicana Volumen 75, núm. 3, A091023, 2023 http://dx.doi.org/10.18268/BSGM2023v75n3a091023
|
 |
First biological inclusion in Upper Cretaceous Texas amber, USA
Primera inclusión biológica en ámbar del Cretácico Superior de Texas, EUA
Paulina Cifuentes-Ruiz1, Virginia Friedman2, Joseph B. Lambert3, George Mustoe4,
Alejandro Bugarin5, Francisco J. Vega6,*
1 Escuela Nacional Preparatoria, Universidad Nacional Autónoma de México.
2 1000 Walnut Place, Mansfield, Texas 76063, USA.
3 Trinity University, Department of Chemistry, San Antonio, Texas 78212, USA.
4 Western Washington University, Geology Department, Bellingham, WA 98225, USA.
5 Florida Gulf Coast University, Department of Chemistry and Physics, Fort Myers, FL 33965, USA.
6 Instituto de Geología. Universidad Nacional Autónoma de México, Ciudad Universitaria, Mexico City 04510, Mexico.
* Corresponding author: (F.J. Vega) This email address is being protected from spambots. You need JavaScript enabled to view it.
How to cite this article:
Cifuentes-Ruiz, P., Friedman, V., Lambert, J. B., Mustoe, G., Bugarin, A., Vega, F. J., 2023, First biological inclusion in Upper Cretaceous Texas amber, USA: Boletín de la Sociedad Geológica Mexicana, 75 (3), A091023. http://dx.doi.org/10.18268/BSGM2023v75n3a091023
Manuscript received: August 11, 2023; Corrected manuscript received: October 9, 2023; Manuscript accepted: October 15, 2023.
ABSTRACT
The first biological inclusion in Cretaceous (Cenomanian) amber from Texas (USA) is here documented. Most of the Cretaceous ambers with biological inclusions are from Europe (Spain, France) and Myanmar (Asia). Although the coleopteran here reported is microscopic and incomplete, it preserves enough morphological details to be identified as a member of the Family Ptinidae Latreille, 1802. This antecedent is significative and reveals the potential of this Cretaceous amber to contain more diverse bioinclusions, since the paleoenvironment suggested by the sediments that contain the amber and the ecological affinity of recent representatives of the Ptinidae suggest a humid forest near an estuary, associated to deltaic plain deposits. Este hallazgo representa la inclusión biológica en ámbar más antigua en las Americas.
Keywords: Coleoptera, Ptinidae, Cenomanian, fossil resin.
RESUMEN
Se documenta la primera inclusión biológica en ámbar del Cretácico (Cenomaniano) de Texas (EUA). La mayoría de los depósitos de ámbar del Cretácico con inclusiones son de Europa (España y Francia) y Myanmar (Asia). Aunque el coleóptero aquí reportado es microscópico e incompleto, preserva suficientes caracteres morfológicos para ser identificado como un miembro de la Familia Ptinidae Latreille, 1802. El presente estudio es significativo y revela el potencial de este ámbar del Cretácico que podría contener bioinclusiones más diversas, dado que el paleoambiente que sugieren los sedimentos que contienen el ámbar y la afinidad ecológica de representantes actuales de Ptinidae, son evidencia de un bosque húmedo asociado a un estero, en la cercanía de la planicie deltáica. This finding represents the oldest inclusion in amber in the Americas.
Palabras clave: Coleoptera, Ptinidae, Cenomaniano, resina fósil.
- Introduction
The first amber-bearing locality has been recently found in North Central Texas (Friedman et al., 2017; 2018) (Figure 1). Subsequent studies of the outcrop assigned the sediments to the Woodbine Group (non-marine Dexter Member) based on stratigraphic, sedimentologic and palynologic data gathered at the locality. The Woodbine Group is an economically important sedimentary unit since it forms significant hydrocarbon reservoirs in the East Texas Basin (Stephenson, 1952). The sediments in the Dexter member are non-calcareous, carbonaceous clays to fine sands. The amber clasts (including the piece containing the first bio-inclusion herein described), were found irregularly distributed on a matrix of finely disaggregated charred wood (fusain). This carbonaceous bed is 15 cm in thickness and has a lateral extent of about 6 feet but is discontinuous in nature. Field work is still in progress and the maximum extent has not yet been determined (Figure 2).
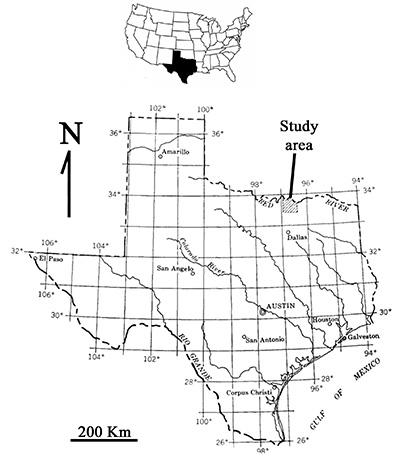 |
| Figure 1. Location map of the study area in North Central Texas, USA. Modified from Friedman et al. (2018). |
The Texas amber has been chemically characterized by 13C and 1H NMR spectroscopy, FT-IR, and GC/ MS. These studies assigned the Texas amber to Group A based on the NMR classification of Lambert et al. (2008) which is equivalent to Class 1b based on the GC/MS and Py-GC/MS classification of Anderson et al. (1992). The botanical source that produced the Texas amber is considered to have been a conifer (Friedman et al., 2018). Further studies indicated a member of the Pinaceae family (Dai et al., 2020). Palynological work at the locality revealed that the paleoflora was composed for the most part of conifers and ferns (among them Pinuspollenites, Deltoidospora). The palynomorphs suggest the presence of a swampy area with nearby conifers and a likely understory of large numbers of ferns.
The palynomorph assemblage, kerogen analysis and sedimentological data indicate a non-marine, fluvial/deltaic environment. The Woodbine Group is a Cenomanian age unit of the Late Cretaceous. Its age (90-100 Ma) was established based on the presence of the ammonite Conlinoceras tarrantense (Kennedy and Cobban, 1990). Based on stratigraphic, sedimentologic, and palynologic data gathered at the locality under study, the age of the Texas amber is assigned to the lowermost Upper Cretaceous (Early Cenomanian) (Friedman et al., 2018). The first biological inclusion was observed microscopically in 2017 by one of us (V.F.) after examining hundreds of amber pieces. It was preliminary reported two years later (Friedman et al., 2019). Other than this, only bacteria and fungi have been tentatively identified in the Texas amber (Girard V. pers. comm.).
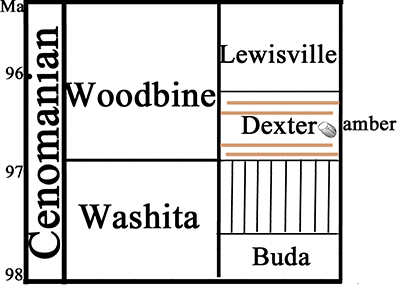 |
| Figure 2. Stratigraphic composed succession of studied area. Modified from Friedman et al. (2018). |
- Paleoenvironment
During the mid-Cretaceous, North America was divided by an inland sea that extended from the Rocky Mountains east to the Appalachians (Sampson et al., 2010). Transgressions and regressions of sea level variations caused changes in shoreline geography and sedimentation rates (Kaufmann, 1984). Floral composition presented also a high degree of variation throughout the Cretaceous. Angiosperms were present in Early Cretaceous plant communities and by Late Cretaceous they were typically a dominant element. As described in the introduction, the Texas amber occurs in association with charcoalified wood. In view of the worldwide presence of charcoal in Cretaceous deposits (Bond and Scott, 2010) the evidence of combustion isn’t surprising. This phenomenon appears to be related to botanical changes. Early angiosperms were short-statured weedy plants that produced an understory more susceptible to combustion compared to the conifer forest. However, this fuel load produced fire intensities that could trigger crown fires in the adjacent forest, resulting in early successional communities that favored rapidly growing angiosperms (Belcher and Hudspeth 2017).
Wildfires may have thus been a contributor to the increasing dominance of flowering plants in late Cretaceous forests. Microscopic examination of the fossilized wood samples collected at the locality where the Texas amber was found revealed the presence of charcoalification. Exterior surfaces of charcoalified wood present a fine, silky luster and a granular texture that is visible at high magnification. This charcoal formation is characteristic of slow-burning fires where oxygen levels are too low to allow complete combustion. Instead, elevated temperatures cause the escape of water and other volatiles from the wood, and reduction of organic matter to pure carbon. It was also noted that in some specimens observed under the SEM, the apparent absence of conductive vessels suggests that the fossil wood is from a gymnosperm.
The low density of charcoal allows fragments to be readily transported by moving water, and it is likely that the amber-bearing sediments were transported by streams from the original habitat area. This hypothesis is supported by the presence of well-rounded sand grains incorporated within the charcoal matrix. The high organic content and fine particle size of the sedimentary material suggests that the final destination was a paludal (e.g., a swamp or marsh). This sediment may represent an accumulation of material derived from a single source, or from multiple streams. The latter possibility is perhaps supported by the presence of amber and charcoalified wood in the same stratum, because the ancient resin would have been susceptible to combustion.
Alternately, the amber may have come from a plant that survived in a forest where only some of the trees perished in a fire. The uncertainty is a result of the fact that amber and charcoal are both relatively buoyant, facilitating fluvial transport. The Dexter member sediments where the Texas amber was found are believed to have been deposited on the flood plains of streams flowing seaward across a coastal plain in early Upper Cretaceous and in fresh- water lakes and lagoons on the coastal plains (Stephenson, 1952).
- Materials and methods
The piece of amber containing the bioinclusion under study was found in the Dexter Member of the Woodbine Group (lowermost Upper Cretaceous, early Cenomanian) in Grayson Co. North Central, Texas, USA. It measures 12 mm length, 8 mm width and 1 mm in thickness of dark yellow translucent amber. The repository of the material is the Friedman-Constantino amber collection, where it has an inventory number of FC-RC-0156.
The amber piece containing the biological inclusion was worked with fine sandpaper and polished with Brasso®. The thickness of the small piece was controlled by looking under an American Optical stereo microscope. The final images were taken with a Zeiss digital camera adapted to a Zeiss Axiozoom.V16, with a PlanNeofluar Z 2.3x objective. The images were taken in series to be processed with Helicon Focus 8, and then edited with Adobe Photoshop Elements 2.0. The classification follows Bouchard et al. (2011).
- Systematic palaeontology
Order Coleoptera Linnaeus, 1758
Suborder Polyphaga Emery, 1886
Series Bostrichiformia Forbes, 1926
Superfamily Bostrichoidea, Latreille, 1802
Family Ptinidae Latreille, 1802
Gen. et sp. indeterminate
Figure 3
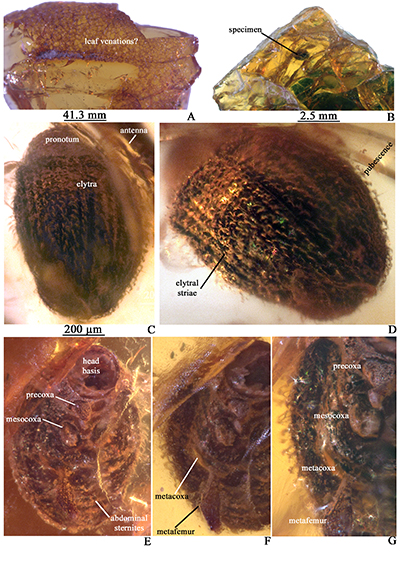 |
| Figure 3. A. Piece of Cenomanian amber from Texas, found along the studied sample, showing posible venations of leaf. B. Amber piece including the small Ptinidae here reported. C. Dorsal view of specimen, showing right antenna, pronotum and elitra covered by pubescence. D. Close-up to elitra, showing striae and pubescence. E. Ventral view showing head basis, precoxa, mesocoxa and abdominal sternites. F. Same view but in different angle to show metacoxa and metafemur. G, Close-up of same view to show precoxa, mesocoxa, metacoxa and metafemur). |
Description. Body ovate, slightly longer than wide (0.95 x 0.74 mm), brown, pubescent. Head incomplete, hypognathous and apparently retracted into pronotum; in ventral view, a relatively large hole (0.16 x 0.17 mm) is visible at its apex. Antenna filiform. Pronotum with convex apical margin. Each elitron has eight striae with two rows of clear decumbent setae.
Precoxae, mesocoxae and metacoxae are visible, with one metafemur and five abdominal sternites.
Comments. The oldest representatives of Ptinidae are known from mid-Cretaceous Burmese amber (Li et al., 2023; Bukejs and Alekseev, 2015). The Cenozoic record for the family is rich, specially for the palaeofauna of the Baltic amber, where 53 species have been described (Bujeks et al. 2021, 2018, 2017; Háva and Zahradník, 2020; Li et al., 2023).
Ecology. Individuals are associated to dead branches and twigs of various trees, and sometimes flowering shrubs. Some members of the family are wood borers and many species feed on accumulated dried animal or plant material of some form (Philips and Bell, 2010).
- Discussion
Some of the visible features in the specimen are typical of members of Ptinidae (e. g. Anobiinae), like the strongly convex shape, the short length, deflexed head inserted into prothorax, deeply covered by pronotum and elytral setae (Philips, 2002). The oldest record of Ptinidae is shared now with a previous one from Cenomanian amber of Myanmar (Bukejs and Alekseev, 2015; Li et al., 2023).
The report of Cretaceous amber with biological inclusions from the Americas represents a significant contribution to the study of fossil biological inclusions, since the most famous amber-bearing lithostratigraphic units are those from the Oligocene-Miocene of Dominican Republic and the early Miocene of Chiapas, Mexico (Solórzano-Kraemer, 2010; Serrano-Sánchez et al., 2016).
Although the insect here reported might represent a transported corpse that got embedded into the fresh resin, the potential to find more terrestrial arthropods in this Cenomanian amber is evident and calls for more intense collection of amber pieces of diverse sizes, since here we demonstrate that the included organisms can be nearly microscopic but with diagnostic features to be reported.
- Conclusion
The first biologic inclusion in Cretaceous (Cenomanian) amber is documented as an incomplete specimen of the coleopteran Family Ptinidae. Previous studies indicate a possible estuary during Cenomanian times, near a deltaic plain environment of the coast of what it is now N Texas (Figure 4), where the amber was deposited. Conifers were the producer of the resin, according to previous reports. The incomplete specimen might have been an incomplete corpse, incorporated into the resin, since if it was a living coleopteran, at least some legs might have been preserved. The absence of the head is an unfortunate detail, due to the breaking of the small piece, just ahead the pronotum.
This finding represents the potential of the Texas amber to preserve other biologic inclusions in what is, until now, the oldest amber with arthropod inclusions in the Americas.
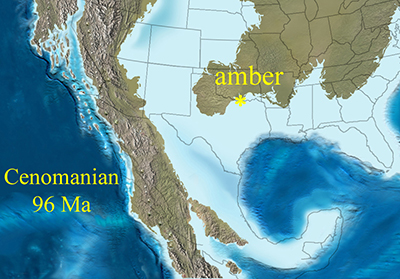 |
| Figure 4. Approximate location of amberiferous area during Cenomanian in what is now North Central Texas. Reproduced with authorization of Ron Blakey. |
Acknowledgements
The authors thank the support of the Welch Foundation (Departmental Grant No. W-0031 to Trinity University Department of Chemistry). We are grateful with the reviewers for their constructive comments.
Contributions of authors
Paulina Cifuentes-Ruiz – Systematic description and identification. Virginia Friedman – material collection and stratigraphic interpretation. Joseph B. Lambert – amber chemical characterization. George Mustoe – paleoenvironmental interpretation. Alejandro Bugarin – amber physical characterization. Francisco J. Vega – preparation of material, illustration of specimens, edition of final manuscript.
Financing
This project was founded by our own resources.
Conflicts of interest
We declare no conflict of interest.
References
Anderson, K.B., Winans, R.F., Botto, R.E., 1992, The nature and fate of natural resins in the geosphere- II. Identification, classification and nomenclature of resinites: Organic Geochemistry, 18(6), 829–841. https://doi.org/10.1016/0146-6380(92)90051-x
Belcher, C.M., Hudspeth, V.A., 2017, Changes to Cretaceous surface fire behavior influenced the spread of early angiosperms: New Phytologist, 213(3), 1521–1532. https://doi.org/10.1111/nph.14264
Bond, W.J., Scott, A.C., 2010, Fire and the spread of flowering plants in the Cretaceous: New Phytologist, 188(4), 1137–1150. https://doi.org/10.1111/j.1469-8137.2010.03418.x
Bouchard, P., Bousquet, Y., Davies, A.E., Alonso-Zarazaga, M.A., Lawrence, J.F., Lyal C.H., Newton, A.F., Reid, C.A.M., Schmitt, M., Ślipińsky, S.A., Smith, A.B., 2011, Family group names in Coleoptera: Zookeys, 88, 1-971. https://doi.org/10.3897/zookeys.88.807
Bukejs, A., Alekseev, V.I., 2015, A second Eocene species of death-watch beetle belonging to the genus Microbregma Seidlitz (Coleoptera: Bostrichoidea) with a checklist of fossil Ptinidae: Zootaxa, 3947(4), 553–562. http://dx.doi.org/10.11646/zootaxa.3947.4.6
Bukejs, A., Alekseev, V.I., Háva, J., 2021, A new species of Xyletinus Latreille, 1809 (Coleoptera: Ptinidae: Xyletininae) from Eocene Baltic amber, with a key to known fossil species: Caucasian Entomological Bulletin, 17(1), 179–184. https://doi.org/:10.23885/181433262021171-179184
Bukejs, A., Bellés, X., Alekseev, V.I., 2018, A new species of Dignomus Wollaston (Coleoptera: Ptinidae) from Eocene Baltic amber: Zootaxa, 4486(2), 195–200. https://doi.org/10.11646/zootaxa.4486.2.9
Bukejs, A., Alekseev, V.I., Cooper, D.M.L., King, G.A., McKellar, R.C., 2017, Contributions to the palaeofauna of Ptinidae (Coleoptera) known from Baltic amber: Zootaxa, 4344(1), 181–188. https://doi.org/10.11646/zootaxa.4344.1.12
Dai, S., Bechtel, A., Eble, C.F., Flores, R.M., French, D., Graham, I.T., Hood, M.M., Hower, J.C., Korasidis, V.A., Moore, T.A., Puttmann, W., Wei, Q., Zhao, L., O’Keefe, J.M.K., 2020, Recognition of peat depositional environments in coal: A Review: International Journal of Coal Geology, 219, 19–56. https://doi.org/10.1016/j.coal.2019.103383
Emery, C., 1886, Über Phylogenie und Systematik der Insekten: Biologisches Centralblatt, 5, 648–656.
Forbes, W.T.M., 1926, The wing folding patterns of the Coleoptera: Journal of the New York Entomological Society, 34(2), 91–139.
Friedman,V., Nguyen, T., Lambert, J.B., 2017, Late Cretaceous Amber in Texas: A preliminary study: Botanical Society of America, Paleobotany Section. Fort Worth, Texas. USA. Poster PPB001, p. 87.
Friedman,V., Lambert, J.B., Contreras, T.A., Stout, E., Kaur, S., Mitamura, H., 2018, Late Cretaceous Amber in Texas: Chemical Characterization and Paleoenvironment: Life: The Excitement of Biology, 5(3), 132–154. https://doi.org/10.9784/leb5(3)friedman.01
Friedman, V., Santiago-Blay, J., Vega, F.J., Serrano, M.deL., Mustoe, G.E., Lambert, J.B., 2019, Upper Cretaceous Texas amber: Its first biological inclusions: 8th International Conference on Fossil Insects, Arthropods and Amber. Santo Domingo, Dominican Republic.
Háva, J., Zahradník, P., 2020, Three new species of Ptinidae (Coleoptera: Bostrichoidea: Ptinidae) from Eocene Baltic amber: Studies and Reports Taxonomic Series, 16(1), 85–91. https://doi.org/10.15298/euroasentj.21.2.07
Kauffman, E.G., 1984, Paleobiogeography and evolution response dynamic in the Cretaceous Interior Seaway of North America, in G.E.G. Westerman (ed.), Jurassic-Cretaceous Biochronology and Paleogeography of North America: Geological Survey of Canada Special Paper 27, 273–306.
Kennedy, W.J., Cobban, W.A., 1990, Cenomanian ammonite faunas from the Woodbine Formation and lower part of the Eagle Ford Group, Texas: Palaeontology, 33(1), 75–154.
Lambert, J.B., Santiago-Blay, J.A., Anderson, K.B., 2008, Chemical signatures of fossilized resins and recent plant exudates: Angewandte Chemie, International Edition, 47, 9608–9616. https://doi.org/10.1002/chin.200910276
Latreille, P.A., 1802, Histoire naturelle générale et particulière des Crustacés et des Insectes. Tome 3. Familles naturelles des genres. Paris. F. Dufart, 467 p. https://doi.org/10.5962/bhl.title.15764
Li, Y.D., Philips, T.K., Huang, D.Y., Cai, C.Y., 2023, Earliest fossil record of Eucradinae in mid-Cretaceous amber from northern Myanmar (Coleoptera: Ptinidae): Bulletin of Geosciences, 98(2), 171–180. https://doi.org/10.3140/bull.geosci.1876
Linnaeus, C., 1758, Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Tomus I. Edito decima, reformata. Holmiae [= Stockholm]: L. Salvii, 824 p. https://doi.org/10.5962/bhl.title.156772
Philips, T.K., 2002, Anobiidae Fleming 1821, in Arnett, R. H. Jr., Thomas, M. C., Skelley, P. E., Frank, J. H. (eds.) Volume 2 American Beetles Polyphaga: Scarabaeoidea through Curculionoidea: CRC Press, p. 245.
Philips, T.K., Bell, K.L., 2010, Ptinidae Latreille, 1802, in Leschen, R.A.B., Beutel, R.G., Lawrence, J.F. (eds.), Handbook of Zoology, Volume 2, Morphology and Systematics (Elateroidea, Bostrichiformia, Cucujiformia partim) (pp. 217–225). De Gruyter. https://doi.org/10.1515/9783110911213.761
Sampson, S.D, Loewen, M.A., Farke, A.A., Roberts,E.M., Forster, C.A., Smith, J.A., Titus, A.L., 2010, New horned dinosaurs from Utah provide evidence for intracontinental dinosaur endemism: PlosOne, 5(9), e12292. https://doi.org/10.1371/journal.pone.0012292
Serrano-Sánchez, M.L., Hegna, T.A., Schaaf, P., Pérez, L., Centeno-García, E., Vega, F.J., 2016, The aquatic and semiaquatic biota in Miocene amber from the Campo La Granja mine (Chiapas, Mexico): Paleoenvironmental implications: Journal of South American Earth Sciences, 62, 243–256. https://doi.org/10.1016/j.jsames.2015.06.007
Solórzano-Kraemer, M.M., 2010, Mexican amber, in Penney, D. (ed.), Biodiversity of fossils in amber from the major world deposits: Manchester, Siri Scientific Press, 42–56.
Stephenson, L.W., 1952, Larger invertebrate fossils of the Woodbine Formation (Cenomanian) of Texas: United States, Geological Survet, Professional Paper, 242, 226. https://doi.org/10.3133/pp242
Peer Reviewing under the responsibility of Universidad Nacional Autónoma de México.
This is an open access article under the CC BY-NC-SA license(https://creativecommons.org/licenses/by-nc-sa/4.0/)

