|
Boletín de la Sociedad Geológica Mexicana Volumen 75, núm. 3, A290623, 2023 http://dx.doi.org/10.18268/BSGM2023v75n3a290623
|
 |
Archaeal communities at gas-venting shallow basins in the northern Gulf of California (Wagner and Consag basins)
Comunidades arqueales en cuencas someras con emisión de gas en el norte del Golfo de California (cuencas de Wagner y Consag)
Fernando Pérez-Villatoro1, Sonia Dávila1, Katy Juárez1, Rosa María Prol-Ledesma2,*
1 Instituto de Biotecnología, Universidad Nacional Autónoma de México, Av. Universidad 2001, 62210, Cuernavaca, Morelos, Mexico.
2 Instituto de Geofísica, Universidad Nacional Autónoma de México, Ciudad Universitaria, 04510, CDMX, Mexico.
* Corresponding author: (R.M. Prol-Ledesma) This email address is being protected from spambots. You need JavaScript enabled to view it.
How to cite this article:
Pérez-Villatoro, F., Dávila, S., Juárez, K., Prol-Ledesma, R.M., 2023, Archaeal communities at gas-venting shallow basins in the northern Gulf of California (Wagner and Consag Basins): Boletín de la Sociedad Geológica Mexicana, 75 (3), A290623. http://dx.doi.org/10.18268/BSGM2023v75n3a290623
Manuscript received: January 16, 2023; Corrected manuscript received: June 27, 2023; Manuscript accepted: June 29,2023.
ABSTRACT
Deep-sea vents microorganisms have been well characterized with defined typical taxonomic groups, principally composed of archaea, while shallow hydrothermal vents are considered to host a different community with bacteria as predominant prokaryotes. This work focuses on two shallow basins in the northern Gulf of California: Wagner and Consag basins which show evidence of early stages of rifting processes and host numerous submarine vents with intense gas discharge. The exploratory study of archaea and bacteria in six sediment samples from the shallow vents in those basins (average depths of 100 m), demonstrate that similar archaea phyla inhabit these vents. The phylum Thermoproteota with Nitrosopumilus as the most abundant genera in five sites. However, in the sample with the highest temperature, the phylum Asgardarchaeota was predominant, and also the occurrence of archaeal lineages typical of deep sea vents like Nanoarchaeota, Thermoproteota, Euryarchaeota, and members from phylum Asgardarchaeota, were identified. This work is the first report of the presence of a typically deep vent community of archaea in this shallow vent environment.
Keywords: shallow submarine vents, extremophiles, Lokiarchaeia, Mexico.
RESUMEN
Los microorganismos que habitan las ventilas submarinas profundas han sido bien caracterizados con grupos taxonómicos típicos bien definidos y están integrados principalmente por arqueas, mientras que se considera que las ventilas someras se caracterizan por una comunidad de microorganismos representados principalmente por bacterias. Este estudio se enfoca en las comunidades de Arqueas en 6 sitios en las Cuencas de Wagner y Consag (con profundidades promedio de 100m), donde se han descubierto evidencias de que existen procesos iniciales de dispersión oceánica y que presentan numerosas ventilas submarinas someras con una intensa descarga de gas. El estudio para identificar la presencia de arqueas y bacterias, usando amplificación y secuenciación de 16S rDNA, se realizó en muestras de sedimento de ambas cuencas y se encontraron phyla de arquea similares a los de ventilas profundas. El phylum Thermoproteota del que Nitrosopumilus fue el más abundante en cinco sitios. Sin embargo, en la muestra proveniente del sitio con la temperatura más alta, el phylum Asgardarchaeota fue el predominante, también se identificaron los típicos de ventilas profundas como Nanoarchaeotas, Thermoproteota, Euryarchaeota, y miembros del phylum Asgardarchaeota. Este trabajo es el primer reporte de la presencia de estas comunidades en este ambiente de ventilas someras.
Palabras clave: ventilas submarinas someras, extremófilos, Lokiarchaeia, México.
- Introduction
Submarine vents are sites where high temperatures and chemical conditions of the expelled fluids favour the occurrence of chemosynthetic communities of archaea and bacteria. The richness and abundance of microbes in deep vents have been studied for several decades and reported in numerous references (Prieur, 1997; Speth et al., 2022; Takai and Nakamura, 2011; Zhou et al., 2022). Specifically, archaea diversity in deep hydrothermal vents has been determined by DNA genome sequences that allowed the identification of thermophilic species of the phyla Thermoproteota and Euryarchaeota as well as methanogens and halophile species (Takai and Horikoshi, 1999; Rinke et al., 2021). In contrast, less attention has been paid to the shallow submarine hydrothermal manifestations with lower temperatures <200°C. Shallow systems are characterized by the presence of a gas phase (absent in the deep-sea), low pressure, sunlight, and photosynthetic rates, which are remarkable differences that distinguish shallow from deep hydrothermal systems (Tarasov et al., 2005). Those conditions define the features of the microbial species present in the shallow vents. Prokaryotic microbial species isolated from shallow hydrothermal vents correspond mainly to archaeal Phyla Thermoproteota and Euryarchaeota, and bacterial phyla Bacteroidetes, Chloroflexi, Firmicutes, Proteobacteria and Thermotogae. These organisms inhabit the subsurface and sediment around hydrothermal emissions, accumulating dense mats that can reach a thickness of up to 30 cm (Tarasov et al., 2005; Rinke et al., 2021).
The most studied shallow venting site is the Baia di Levante of Vulcano Island (Eolian Islands), where abundant information has been produced on the microbial communities of bacteria and archaea (Fiala & Stetter, 1986; Stetter, 1988; Caccamo et al. 2001; Maugeri et al., 2001, 2002, 2009, 2010; Gugliandolo et al. 2003; Gugliandolo and Maugeri, 2019; Rizzo et al., 2022). The microorganisms reported include aerobic and anaerobic, thermophilic, and hyperthermophilic species. Archaea identified in Panarea vents are similar in community structure to those collected from submarine vents and geothermal wells in New Zealand and nearby Volcano Island (Maugeri et al., 2009). A report for the Flegrean area, at Cape Palinuro and around the Eolian Islands (Maugeri et al., 2010) confirms that the richness and abundance of bacteria is greater than for archaea, as observed in most shallow vent systems. Recent studies of bacterial communities in a low-temperature shallow vent site revealed the predominance of proteobacteria and bacteroidetes (Rajasabapathy et al., 2018).
The second most studied area contains the shallow vents near Milos Island, where archaea represent only a minor part of the microbial community (Sievert et al., 2000). On the other hand, in the shallow vents at the Kodakara-Jima Island in Japan, Hoaki et al. (1994) reported the presence of hyperthermophilic archaea closely related to the genus Thermococcus.
The 200-210 m deep Wagner and Consag Wagner and Consag Basins basins are the northernmost of the 8 active extensional basins within the Gulf of California (Figure 1). This is a particular zone where active rifting and intense gas discharge recently have been discovered, related with anomalously high heat flow (Prol-Ledesma et al., 2013); the presence of extensive gas venting and heated sediments along the main faults indicates an estimated 15,000 CO2-containing gas vents, each up to 100 m wide and giving rise to water column pH values as low as 6.3. This range of pH values and the higher than average sediment temperature in the studied area make this an excellent area for studying the combined effects of high temperature and low pH on microbial communities. In this work, we report the results of the characterization, using the 16S rRNA gene clone library approach, of the archaeal community found in the sediments from the Wagner and Consag Basins, in order to gain new insight into the archaeal communities of shallow vents.
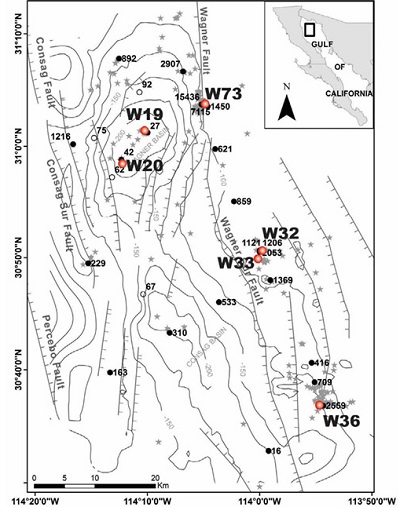 |
| Figure 1. Bathymetric map and heat flow data of the Wagner and Consag basins; jagged lines denote faults, stars indicate vent location (after Prol-Ledesma et al., 2013). Numbers indicate heat flow data (values in mWm-2, the mean ocean heat flow is 105.4mWm-2). Sediment sampling sites shown with red dots and the station number: W19, W20, W32, W33, W36, W73, four of them are located nearby the largest vents in Figure 1 (W32, W33, W36, W73-highest heat flow values), site W73 is close to a mud volcano and stations W19 and W20 are in the Wagner Basin bottom with no evident features of gas discharge (low heat flow values). |
- LOCATION AND CHARACTERISTICS OF THE SAMPLING SITES
The Gulf of California is an example of the transition from continental rift to seafloor spreading. The extensional tectonics in the area separated the Baja California Peninsula from the North American plate by about 6 Ma, forming a series of basins separated by transformed faults related to the San Andreas fault system. There are 8 basins where active extensional features have been documented. Spreading centres maturity decreases towards the north, and new oceanic crust has been positively identified only in the southernmost basins (Lizarralde et al., 2007; Paduan et al, 2018); therefore, the studied area represents a nascent ocean basin where hydrothermal activity is in its early stages. The studied basins (Wagner and Consag) had not been considered active spreading centres (Persaud et al., 2003) until recently, when heat flow and seismic evidence revealed the occurrence of active spreading features and incipient hydrothermal manifestations (González-Escobar et al., 2010; Prol-Ledesma et al., 2013; Neumann et al., 2017).
The shallow Wagner and Consag basins (200-210 m depth) have a large sedimentation rate that accounts for a great thickness of the sedimentary cover of about 7 km. In this area, mixing in the water column results in a homogeneous temperature of 15 °C at the sea bottom that remains practically constant the whole year (Álvarez-Borrego and Lara-Lara, 1991) except at the hydrothermal vent discharge, where temperatures higher than 60 °C were measured at some vents (Prol-Ledesma et al., 2013). The mean heat flow in the area is approximately 2 W/m2, which is about 20 times the average seafloor heat flow (0.105 W/m2; Davies and Davies, 2010). The highest heat flow values are aligned with the Wagner fault that is the most relevant structure where the largest vents were discovered, and four sampling sites are positioned (Figure 1).
Seismic studies provide evidence of deep intrusions at the base of the sedimentary cover (González-Escobar et al., 2010). Analyses of the gas sample showed that the gas from the sediment was predominantly CO2 and methane (Prol-Ledesma et al., 2013). Some of the deepest water samples collected within the plumes had Ba, As and Mn concentrations (30, 60, 12 µg/l, respectively, 20 m above the seabed), around 3 times higher than background (Canet et al., 2010). The presence of extensive gas venting and heated sediments along the main faults indicates an estimated 15,000 CO2-discharging gas vents, giving rise to water column pH values as low as 6.3 (Canet et al., 2010). This range of pH values and the higher than average sediment temperature are expected to generate a specific ecosystem including a distinct microbial community. Biological studies in this area include benthic Foraminifera, Sipunculida and Kinorhyncha distribution in relation with venting, their abundance seems to be related with the gas venting occurrence and geochemistry (Hermoso-Salazar et al., 2013; Pettit et al., 2013; Álvarez-Castillo et al., 2015, 2018, 2023). Twelve higher taxa were recorded in both basins, where meiofauna was dominated by Nematoda (73.1%) followed by Copepoda Harpacticoida (11.28%), Polychaeta (8.41%) and Kinorhyncha (4.71%) (Álvarez-Castillo et al., 2018). Close to fluid outlets in the softer sediments, there was a community of chaetopterid species, with tubes up to 40 cm or more in length and one station contained 3 specimens of a bivalve Solemya (Petrasma) sp. with endosymbiotic bacteria in the gills (Canet et al., 2010).
Organic matter makes up to 2% in the sediment samples and more than 20% is bitumen, which indicates a high level of organic matter maturation (Ángeles et al., 2017). None of the recovered samples showed signs of hydrocarbon staining; however, evidence of the presence of hydrothermal activity in the Wagner and Consag basins is provided by the SOM alteration. The hydrothermal activity in the area generates the maturation of organic matter, as inferred from the presence of diverse compounds: polycyclic aromatic hydrocarbons (PAHs), mono and dimethylated alkanes, trimethylnaphthalene isomers and their relative proportions in the sediment samples (Ángeles et al., 2017). The identification of the pentamethylicosane (PMI) isoprenoid in sediment samples from the Wagner- Consag basins indicated the probable presence of Thermoproteota that live at temperatures between 75 and 105°C (Thiel et al., 1999; Madigan et al., 2003), this is important evidence of the occurrence of hydrothermal activity even when the main manifestation is intense gas venting.
- Methodology
3.1. SAMPLE COLLECTION AND CHEMISTRY
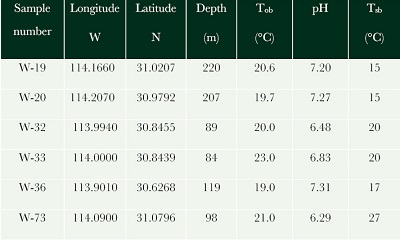
During the WAG-2 cruise of the O/V “El Puma” the seabed sediments of the Wagner and Consag Basins were sampled using a USNEL box corer and a Smith-McIntyre grab in 6 sites within and outside the basins (Figure 1). Temperature profiles were measured within the sediments to determine temperature gradient using a 6m long FIELAX probe with 11 thermistors, these measurements included sea bottom temperature determination (Tsb). Additionally, the temperature of recovered sediment was immediately measured on board with a digital thermometer on recovery (Tob). The pH was immediately measured on the water samples using a pH meter with an accuracy of 0.01 pH units and compared with buffers prepared in artificial seawater (Table 1).
| Table 1. Sample location, Tsb-sea bottom temperature, Tob-on board sample temperature. |
 |
3.2. DNA EXTRACTION, CLONING AND SEQUENCING OF 16S RDNA GENES
Total DNA was extracted using the Power Soil® DNA Isolation Kit (Mo Bio). 16S rDNA genes were amplified using the universal 16S primers for bacteria 27F and 1492R (Lane, 1991), and A21F (5‘-TTCCGGTTGATCCYGCCGGA-3’) and A958R (5’-YCCGGCGTTG AMTCCAATT-3’) for archaea. 1492R primer (5′-TACCTTGTTACGACTT) and one of the following three 27f primer formulations: twofold-degenerate primer 27f-CM (5′-AGAGTTTGATCMTGGCTCAG, where M is A or C), fourfold-degenerate primer 27f-YM (5′-AGAGTTTGATYMTGGCTCAG, where Y is C or T), The reaction mixtures were incubated at 94°C for 4 min, followed by 33 cycles of denaturation at 94°C for 30 s, annealing at 54°C for 15 s, and extension at 72°C for 45 s.
Purified PCR products were cloned into TOPO TA cloning vector pCR2.1 according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA) and transformed into E. coli XL1-Blue competent cells by electroporation. Transformants were transferred to plates containing LB broth (25 ug/ml kanamycin and 200 ug/ml ampicillin) and grown overnight at 370C. Plasmids were isolated using High Pure Plasmid kit (ROCHE, Indianapolis, IN); subsequently, clones were digested (2 h, 370C) with EcoR1 (BioLabs, New England) for evaluating the presence of inserts. Inserts were sequenced at the Sequencing Facility of the Instituto de Biotecnologia, Universidad Nacional Autonoma de Mexico, using a Taq FS Dye Terminator cycle fluorescence-based sequencing with an automated capillary sequencer (Perkin Elmer, model 3130x1, Applied Biosystems).
3.3. 16S RDNA SEQUENCES AND PHYLOGENETIC ANALYSIS
Forward and reverse sequences were pre-processed and assembled using free available software (Staden et al, 2003). Potential chimeric sequences were identified using the software UCHIME (Edgar et al, 2011) and DECIPHER (Wright et al., 2012). The sequences identified by BLAST (available through the National Center for Biotechnology Information) as 16S rRNA were subsequently aligned using ClustalW (Thompson et al., 1994). Aligned sequences having >97% of similarity were clustered into operational taxonomic units (OTUs) by the average neighbour algorithm (Legendre and Legendre, 1998), for each OTU the sequence with the smallest maximum distance to the other sequences (representative sequence) was selected using Mothur (Schloss et al., 2009). The representative sequence for each OTU was taxonomically assigned using the SILVA 128 Incremental Aligner (SINA) Online (Pruesse et al., 2012; Quast et al., 2013). Phylogeny was inferred using the Maximum Likelihood method based on the Kimura 2-parameter model (Kimura, 1980). the consensus phylogenetic tree was inferred from 500 bootstrap replicates, evolutionary analyses were conducted in MEGA6 (Tamura et al., 2013).
Bacterial community was inspected, after raw sequence clean and processing there were obtained 38 bacterial 16S rRNA sequences with an average length of 756bp. The number of sequences for each sampled site ranged from 2 to 12. All the sequences were average neighbour clustering at a 0.03 distance level, in total 12 OTUs were produced. After clustering independently each sampled site’s sequences 2 to 5 OTUs were produced. The 12 OTUs were taxonomically classified. Data available in https://www.ncbi.nlm.nih.gov/popset/1345460006 , https://www.ncbi.nlm.nih.gov/popset/1343668055
- Results
4.1. ARCHAEA DIVERSITY
The 41 OTUs were taxonomically classified (Figure 3); OTU1 and OTU2 are the most abundant OTUs with 48% of all sequences, those were affiliated to the candidate Nitrosopumilus maritimus from Thermoproteota Phylum, this archaea is capable to grow in environments with very low ammonium concentrations and is ubiquitous in aquatic ecosystems (Offre et al, 2013). The high abundance of Nitrosopumilus maritimus in all the sampling sites may correspond to the predominance of seawater content in the sediments. Two OTUs could not be associated with any archaeal phyla and may belong to some novel archaeal phylum. The rest of the OTUs were classified into the novel proposed phyla: Asgardarchaeota, Thermoproteota and Nanoatchaeota; to the uncultivable classes like, Nitrosospaeria from the phylum Thermoproteota, Thermococci from Euryarchaeota and Lokiarchaeia from the Asgardarchaeota phylum, all those archaeal lineages have previously been reported in marine sediments and near to deep marine hydrothermal vents (Takai and Horikoshi, 1999; Zaremba-Niedzwiedzka et al., 2017; MacLeod et al., 2019; Rinke et al., 2021).
The relative abundance of the identified archaeal lineage for each sampled site is shown in Figure 3. About 50 % of the sequences were taxonomically assigned as Nitrosopumilos maritimus (phylum Thermoproteota), such microorganisms are one of the most common archeon living in seawater. Two OTUs could not be associated with any archaeal phylum and may belong to some novel archaeal phyla (Figure 2). The rest of the OTUs were classified into the novel proposed phylum: Asgardarchaeota, Thermoproteota and Nanoarchaeota; to the uncultivable Group C3 Thaumarcheota and the candidate’s divisions WSA2 and Marine Hydrothermal Vent Group (MHVG), all these archaeal lineages have previously been reported in marine sediments and near to deep marine hydrothermal vents (Adam et al., 2017; Spang et al., 2017).
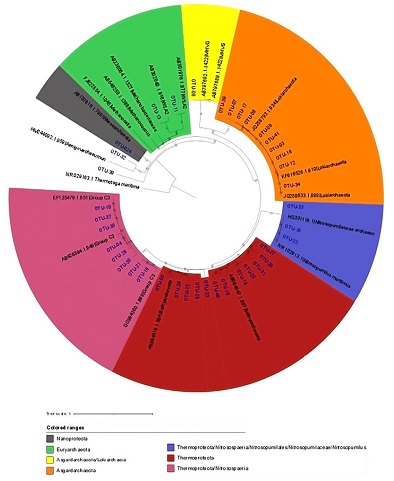 |
| Figure 2.Phylogenetic tree of the archaeal sequences clones. The representative sequence for each archaeal OTU were aligned by ClustalW. Phylogeny was inferred using the Maximum Likelihood method based on the Kimura 2-parameter model, the consensus phylogenetic tree was inferred from 500 bootstrap replicates. |
Asgardarchaeota was the second most abundant phylum identified, many of these archaea were previously classified as Marine Benthic Group B (Solden et al., 2016); this group is related to the iron cycle (Aoki et al., 2014) and some archaea of this group have been also found in marine sediments (Durbin and Teske, 2012; Fry et al., 2008; Hugoni et al., 2015; Jiao et al., 2011; Na et al., 2015; Teske et al., 2002; Teske and Sorensen, 2008; Rinke et al., 2021). In this study, the presence of Asgardarchaeota was observed in all sites; however, Asgardarchaeota was the most abundant archeon in site W33 (Figure 3), at approximately 80 m depth, where the most intensive gas discharge and extremely high heat flow were detected (Figure 1).
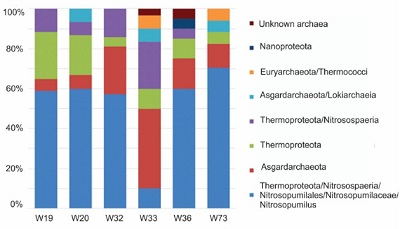 |
| Figure 3. The plot presents the relative abundance of each identified archaeal lineage for each sampled site. The representative sequence for each OTU was taxonomically assigned using SILVA 128 Incremental Aligner (SINA) Online (Pruesse et al., 2012; Quast et al., 2013). |
The third most abundant phylum identified was Thermoproteota previously named Miscellaneous Crenarchaeota Group (Meng et al., 2014; Rinke et al., 2021), this archaeal group has capabilities for protein fermentation (Lloyd et al., 2013; Meng et al., 2014; Rinke et al., 2021), homoacetogenesis (Lazar et al., 2016; Meng et al., 2014) and methane cycling (Evans et al., 2015). The fourth most abundant archaeal lineage identified was the Thermoproteota phylum Nitrosospaeria class, in many reports identified as dominant phyla in deep hydrothermal vents, and also abundant in W33 (Nunoura et al 2018; Meng et al., 2014; Rinke et al., 2021).
Archaeal lineages Lokiarchaeia class and Thermococci class were less abundant and were present in two sites W33 and W73 (Figure 3). Lokiarchaeia class is a deep branch archaeal lineage detected near to deep marine hydrothermal vents and has been poorly described (Teske and Sorensen, 2008). Members of Nanoarchaeota, a phylum which was previously named Deep-sea Hydrothermal Vent Euryarchaeota Group-6, might primarily be involved in anaerobic carbon cycling and is considered to have a symbiotic lifestyle (Castelle et al.,2015; Rinke et al., 2021), it was present only in site W36 (Figure 3). The Thermococci class archaea have been detected in a wide range of natural environments including deep marine hydrothermal sediments (Nobu et al.,2016 ; Rinke et al., 2021). Whole genome sequencing shows that these archaeal groups possess metabolic routes for methanogenesis (Dhillon et al, 2005). In this study its occurrence was restricted to two sites W33 and W73 (Figure 3), where the most intense gas discharge occurs.
The appearance of some lineages like Nitrosopoeria class from the Thermoproteota phylum and Nanoarchaeota phylum (typically dominant archaea groups in deep hydrothermal vents) in the shallow vents studied; suggest that fluid chemistry could be one of the most important factors determining their presence in these environments, possibly more restricted by local temperature and fluid composition than by depth and pressure.
4.2. BACTERIA DIVERSITY
The identified Bacterial taxonomic clones for each sampled site are presented in Table 2. The Phylum Proteobacteria was found to be dominant with presence in all sites studied, Class Gammaproteobacteria (Teske and Salman, 2014) was the most abundant bacterial lineage identified to correspond to the genera Psychrobacter, Shewanella and Amphritea; bacteria that belong to these genera have previously been detected near to marine hydrothermal vents (Gärtner et al., 2008; Sun et al., 2015; Zhou et al., 2016; Satomi, 2014).
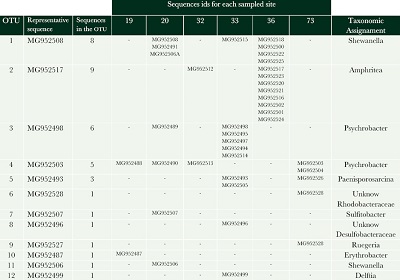
| Table 2. Bacterial taxonomic clones identified per sampled site. The sequences from all sampled sites were average neighbour clustered, and the description of the number of sequences per OTU, sequence id and taxonomic assignment is shown. |
 |
- Discussion
The formation of typical chimneys associated with hydrothermal activity at the sea bottom has not been documented in the Wagner and Consag basins. However, there is evidence of convective hydrothermal circulation based on the extremely high heat flow that was measured in the Wagner Basin (Prol-Ledesma et al., 2013). Additionally, extensive gas discharge was observed and reported in the area, and the predominance of CO2 and CH4 was measured. The discharge of CO2 is common in hydrothermal systems and in this case it is associated with the ocean acidification measured in this area, and the presence of CH4 is frequently related with methanotrophs presence.
The results of this study present the composition of archaeal and bacterial communities at 6 different sites in an area characterized by incipient hydrothermal activity related with ocean spreading. Four samples represent the sites with the most intense fluid discharge and high heat flow, and two sites were sampled where flares have not yet been detected. A total of 120 archaeal sequences using amplification and sequencing of 16S rDNA gene were obtained. About 50 % of the sequences were identified as Nitrosopumilus maritimus (Phylum Thermoproteota, class Nitrosospaeria), such microorganisms are one of the most common archaeon living in seawater. The rest of the OTUs were classified into the novel proposed phyla: Asgardarchaeota, Thermoproteota and Nanoarchaeota; and into the uncultivable classes like, Nitrosospaeria from the phylum Thermoproteota, Thermococci from Euryarchaeota phylum and Lokiarchaeia from the Asgardarchaeota phylum, all these archaeal lineages have previously been reported in marine sediments and near to deep marine hydrothermal vents (Teske and Sorensen, 2008). The presence of Asgardarhaeota in shallow vents indicates that they are more adaptable and not restricted exclusively to deep hot vents. High temperature seems to favour their abundance as the site where they were most abundant is one with strong venting activity. Apparently, the high temperature and intense venting activity boost up its higher relative abundance in sites W32, W33, W36 and W73, compared to their low abundance in the inactive sites W19 and W20.
The recently discovered candidate Archaeal Phylum Asgardarchaeota was defined previously as part of the Deep Sea Archaeal Group (DSAG) and it is a likely candidate to stand as a link with the Eukaryotic domain with which they share specific genome features (Spang et al., 2015; Rinke et al., 2021). In this work we show that within the sediments affected by hydrothermal shallow vents, Asgardarchaeota represents an important part of the microbial composition and diversity and is the dominant phyla in the hottest sample at a depth of approximately 80m. The presence of Asgardarchaeota in shallow vents, indicates that they are more adaptable and not restricted exclusively to deep hot vents. Zaremba-Niedzwiedzka et al. (2017) reported a wide distribution of what they denominated the Aasgard clade in different environments; nonetheless, they did not find any representatives of the Aasgard clade in the shallow vent site that they studied.
The similarity of some groups like Thaumarchaeota in shallow and deep vents indicate that depth/pressure may not play a key role in the microbial diversity, and that fluid chemistry and temperature are the main factors in the species present in the shallow and deep sea bottom hydrothermal vents.
Recent studies demonstrated that the growth rate of Archaeoglobus fulgidus is not affected up to pressures equivalent to 4000 m depth (Oliver et al., 2020); however, further work is required to evaluate the optimal pressure for the different phyla. Presently, the presented evidence suggests that deep-sea archaea occurrence is more restricted by temperature than by pressure.
In the case of bacteria, Psychrobacter and Shewanella genera have been detected in a wide range of marine habitats (Teske and Sorensen 2008; Satomi, 2014) and the Amphritea genus is closely associated with living marine organisms (Jang et al., 2015).
Bacterial families Rhodobacteraceae and Planococcaceae and the genera Erythrobacter, Delftia and Sulfitobacter were identified in less abundance. The clones that belong to the families Rhodobacteraceae and Planococcaceae may correspond to novel bacteria genera, both Planococcaceae and Rhodobacteraceae bacteria have been detected in a wide variety of habitats (Pujalte et al., 2014; Shivaji et al., 2014). The genera Erythrobacter, Delftia and Sulfitobacter also have been detected in a wide range of habitats; therefore, it is expected that the physiology of each genera species should be diverse (Rosenberg et al., 2013).
The shallow vents in two sites in western Mexico, in Bahía Concepción and Punta Mita where microbial communities have been characterized, present different redox conditions that constrain the presence/absence of distinct species of bacteria: Gamma-, Delta-, and Epsilonproteobacteria as well as Bacteroidetes are present under the oxidizing conditions of Bahía Concepción, and Thermotogae, Aquificae, and Planctomycetes were identified in the shallow vents under strongly reduced conditions in Punta Mita (Dávila-Ramos et al., 2015).
One of the best documented shallow hydrothermal areas is the Baia di Levante of Vulcano Island (Eolian Islands), where abundant information has been produced about the bacteria and archaea communities present on that site (Caccamo et al., 2001; Fiala and Stetter, 1986; Gugliandolo et al., 2003; Maugeri et al., 2001, 2009; Segerer et al., 1986; Stetter, 1988); the microorganisms reported include aerobic and anaerobic, thermophilic, and hyperthermophilic species. In the shallow vents near Milos Island, archaea represent less than 10% in average of the microbial community (Sievert et al., 2000), similarly to what was observed in shallow vents from Panarea, Italy and western Mexico (Dávila-Ramos et al., 2015; Lentini et al., 2014). In Panarea vents, the archaea identified are less abundant than bacteria and restricted to the hottest areas in this system (Euryarchaeota: Thermococci and Thermoplasmata; and Crenarchaeota: Thermoprotei) (Lentini et al., 2014). The studies of microbial communities in shallow vents generated by serpentinization reactions like Prony Bay, reveal low archaeal diversity with a predominance of methane metabolizing organisms, very similar to the diversity observed in deep vents with the same processes of serpentinization (Quéméneur et al., 2014). Therefore, the presence of archaea and bacteria in the shallow vents studied here extends the conditions under which these communities have been reported.
- CONCLUSIONS
In this work, we demonstrated the presence of archaea, typically associated with deep-sea hydrothermal vents, in sediments from shallow vents corresponding to the Wagner and Consag basins in the northern Gulf of California. We suggest that discharge and composition of hydrothermal fluids and local temperature, more than depth, are the determinant factors favouring the occurrence of specific microbial groups like Asgardarchaeota and Nanoarchaeota (previously named Deep-sea Hydrothermal Vent Euryarchaeota Group) and typically associated to deep sea vents and now also detected in the shallow vents studied. This work confirms the presence of the deep vent community of archaea and the Lokiarchaeia class of the Asgardarchaeota phylum in shallow hydrothermal vents.
The study of microbial communities in sediment samples from the shallow vents in the Wagner and Consag basins demonstrated that these archaea and bacterial communities are similar to those identified in deep sea hydrothermal vents. Altogether, these findings represent valuable information for understanding the microbial distribution and potential ecological roles in deep and shallow hydrothermal environments.
Author contribution
- conceptualization: RMPL, KJ; (2) analysis/data acquisition: FPV, SD; (3) methodological development: KJ, FVP, SD; (4) writing original manuscript: KJ, FVP, RMPL, SD; (5) writing revised manuscript: KJ, FVP, RMP, SD; (6) graphic design: FPV, SD; (7) field work: RMPL; (8) interpretation: KJ, FVP, RMP, SD; (9) funding: RMPL.
Funding
This research was funded jointly by Mexico and the European Union through the project FONCICYT 94482.
Acknowledgements
This research was funded jointly by Mexico and the European Union through the project FONCICYT 94482. We want to thank the Coordinación de Plataformas Oceánicas (COPO, UNAM). Thanks are due to the crew of the “El Puma” Research Vessel of the UNAM for their assistance, and also to José Guadalupe Gómez López and Fernando Sandoval Medina, who helped during sampling. Ezequiel Tobon for technical assistance and sediment sampling and preparation of samples was performed by Alejandro Estradas.
Conflict of Interest
The authors declare no financial/commercial conflict of interest.
References
Adam, P.S., Borrel, G., Brochier-Armanet, C., Gribaldo, S., 2017,The growing tree of Archaea: new perspectives on their diversity, evolution and ecology: The ISME Journal 11, 2407-2425. https://doi.org/10.1038/ismej.2017.122
Álvarez-Borrego, S., Lara-Lara, J. R., 1991, The physical environment and primary productivity of the Gulf of California, in Dauphin, J. P. and Simoneit, B. R. (eds.), The Gulf and peninsular provinces of the Californias: AAPG Mem., 47, 555–567.
Álvarez-Castillo, L., Hermoso-Salazar, M., Estradas-Romero, A., Prol-Ledesma, R.M., 2015, Kinorhyncha from the Gulf of California: horizontal and vertical distribution of four genera in shallow basins with CO2 venting activity: CBM-Cahiers de Biologie Marine, 56, (3), 271-281.
Álvarez-Castillo, L., Hermoso-Salazar, M., Estradas-Romero, A., Rivas, G., Prol-Ledesma, R.M., 2018, Composition and spatial distribution of the meiofauna in the Wagner and Consag basins, Gulf of California, Mexico: CBM-Cahiers de Biologie Marine, 59, 245-256. https://doi.org/10.21411/CBM.A.ACD5ED1C
Álvarez-Castillo, L., Hermoso-Salazar, M., Rivas, G., Estradas-Romero, A., Prol-Ledesma, R.M., 2023, Effect of high temperature on the meiofauna composition, abundance, and biomass in a zone of geophysical anomalies: Marine Ecology, e12751. https://doi.org/10.1111/maec.12751
Ángeles, C., Prol-Ledesma, R.M., Flores-Castro, K., 2017, Organic matter characterization in sediments from the Wagner-Consag Basins, Gulf of California: evidence of hydrothermal activity: Procedia Earth and Planetary Science, 17, 550-553. https://doi.org/10.1016/j.proeps.2016.12.139
Aoki, M., Ehara, M., Saito, Y., Yoshioka, H., Miyazaki, M., Saito, Y., Miyashita, A., Kawakami, S., Yamaguchi, T., Ohashi, A., Nunoura, T., Takai, K., and Imachi, H., 2014, A long-term cultivation of an anaerobic methane-oxidizing microbial community from deep-sea methane-seep sediment using a continuous-flow bioreactor: PLoS ONE, 9(8), e105356. https://doi.org/10.1371/journal.pone.0105356
Caccamo, D., Maugeri, T.l., Gugliandolo, C., 2001, Identification of thermophilic and marine bacilli from shallow thermal vents by restriction analysis of their amplified 16S rDNA: Journal of Applied Microbiology, 91, 520–524. https://doi.org/10.1046/j.1365-2672.2001.01410.x
Canet, C., Prol-Ledesma, R.M., Dando, P., Vázquez-Figueroa, V, Shumilin, E., Birosta, E., Sánchez, A., Robinson, C.J., Camprubí, A., Tauler, E., 2010, Discovery of Massive Seafloor Gas Seepage along the Wagner Fault, Northern Gulf of California: Sedimentary Geology, 228, 292–303. https://doi.org/10.1016/j.sedgeo.2010.05.004
Castelle, C. J., Wrighton, K.C., Thomas, B.C., Hug, L.A., Brown, C.T., Wilkins, M.J., Frischkorn, K.R., Rtinge, S.G., Singh, A., Markillie, L.M., Taylor, R.C., Williams, K.H., Banfield, J.F., 2015, Genomic Expansion of Domain Archaea Highlights Roles for Organisms from New Phyla in Anaerobic Carbon Cycling: Current Biology , 25 , 690 – 701. https://doi.org/10.1016/j.cub.2015.01.014
Dávila-Ramos, S., Estradas-Romero, A., Prol-Ledesma, R.M., Juárez-López, K., 2015, Bacterial populations (first record) at two shallow hydrothermal vents off Mexican Pacific west coast: Geomicrobiology Journal, 32(8), 657-665. https://doi:10.1080/01490451.2014.980526
Davies, J.H., Davies, D.R., 2010, Earth’s surface heat flux: Solid Earth, 1, 5–24. http://dx.doi.org/10.5194/se-1-5-2010
Dhillon, A., Lever, M., Lloyd, K.G., Albert, D.B., Sogin, M.L., Teske, A., 2005, Methanogen diversity evidenced by molecular characterization of methyl coenzyme M reductase A (mcrA) genes in hydrothermal sediments of the Guaymas Basin: Appl Environ Microbiol 71, 4592–4601. https://doi.org/10.1128%2FAEM.71.8.4592-4601.2005
Durbin, A.M., Teske, A., 2012, Archaea in organic-lean and organic-rich marine subsurface sediments: an environmental gradient reflected in distinct phylogenetic lineages: Frontiers in Microbiology, 3, 168. https://doi.org/10.3389/fmicb.2012.00168
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C., Knight, R. 2011, UCHIME improves sensitivity and speed of chimera detection: Bioinformatics, 27, 2194-2200. https://doi.org/10.1093/bioinformatics/btr381
Evans, P.N., Parks, D.H., Chadwick, G. L., Robbins, S. J., Orphan, V. J., Golding, S. D., Tyson, G. W., 2015, Methane metabolism in the archaeal phylum Bathyarchaeota revealed by genome-centric metagenomics: Science, 350, 434-438. https://doi.org/10.1126/science.aac7745
Fiala, G., Stetter, K.O., 1986, Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100ºC: Archives of Microbiology, 145, 56–61. https://doi.org/10.1007/BF00413027
Fry, J. C., Parkes, R. J., Cragg, B. A., Weightman, A. J., Webster, G., 2008, Prokaryotic biodiversity and activity in the deep subseafloor biosphere: FEMS Microbiology Ecology, 66, 181–196. https://doi.org/10.1111/j.1574-6941.2008.00566.x
Gärtner, A., Wies,e J., Imhoff, J.F., 2008, Amphritea atlantica gen. nov., sp. nov., a gammaproteobacterium from the Logatchev hydrothermal vent field: International Journal of Systematic and Evolutionary Microbiology, 58, 34–39. https://doi.org/10.1099/ijs.0.65234-0
González-Escobar, M., Suárez-Vidal, F., Hernández-Pérez, J.A., Martín-Barajas, A., 2010, Seismic reflection-based evidence of a transfer zone between the Wagner and Consag basins: implications for defining the structural geometry of the northern Gulf of California: Geo-Marine Letters 30, 575–584. https://doi.org/10.1007/s00367-010-0204-0
Gugliandolo, C., Maugeri, T.L., Caccamo, D., Stackebrandt, E., 2003, Bacillus aeolius sp. nov. a Novel Thermophilic, Halophilic Marine Bacillus Species from Eolian Islands (Italy): Systematic and Applied Microbiology. 26, 172–176. https://doi.org/10.1078/072320203322346001
Gugliandolo, C., Maugeri, T.L., 2019, Phylogenetic diversity ofarchaea in shallow hydrothermal vents of Eolian Islands, Italy: Diversity, 11, 156-175. https://doi.org/10.3390/d11090156
Hermoso-Salazar, M., Frontana-Uribe, S., Solís-Weiss, V., Prol-Ledesma, R.M., Estradas-Romero, A., 2013, The occurrence of Sipuncula in the Wagner and Consag Basins, Northern Gulf of California: CBM-Cahiers de Biologie Marine. 54, 325-334. https://dx.doi.org/10.21411/CBM.A.FF628B41
Hoaki, T., Nishijima, M., Kato, M., Adachi, K., Mizobuchi, S., Hanzawa, N., Maruyama, T., 1994, Growth Requirements of Hyperthermophilic Sulfur-Dependent Heterotrophic Archaea Isolated from a Shallow Submarine Geothermal System with Reference to Their Essential Amino Acids: Applied and Environmental Microbiology, 60, 2898-2904. https://doi.org/10.1128/aem.60.8.2898-2904.1994
Hugoni, M., Domaizon, I., Taib, N., Biderre-Petit, C., Agogue, H., Galand, P. E., Debroas, D., Mary, I., 2015, Temporal dynamics of active archaea in oxygen depleted zones of two deep lakes: Environmental Microbiology Reports, 7, 321–329. https://doi.org/10.1016/j.resmic.2013.01.004
Jang, H., Yang, S. H., Seo, H. S., Lee, J. H., Kim, S. J., Kwon, K. K. 2015, Amphritea spongicola sp. nov., isolated from a marine sponge, and emended description of the genus Amphritea: International Journal of Systematic and Evolutionary Microbiology, 65(6), 1866-1870. https://doi.org/10.1099/ijs.0.000188
Jiao, L., Chen, F., Zhang, Y., 2011, Microbial diversity in sediments of core HS-PC 500 from Shenhu area, northern south China Sea: Acta Microbiologica Sinica, 51,876-890.
Kimura, M., 1980, A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences: Journal of Molecular Evolution, 16, 111–120. https://doi.org/10.1007/bf01731581
Lane, D.J., 1991, 16S/23S rRNA sequencing, in Stackebrandt, E., and Goodfellow, M., (ed.), Nucleic acid techniques in bacterial systematics: New York, John Wiley & Sons, 115-175.
Lazar, C. S., Baker, B. J., Seitz, K., Hyde, A. S., Dick, G. J., Hinrichs, K. U., Teske, A. P., 2016, Genomic evidence for distinct carbon substrate preferences and ecological niches of Bathyarchaeota in estuarine sediments: Environmental Microbiology, 18,1200-1211. https://doi.org/10.1111/1462-2920.13142
Legendre, P., Legendre, L., 1998, Numerical ecology: New York, Elsevier, 990p.
Lentini, V., Gugliandolo, C., Bunk, B., Overmann, J., Maugeri, T.L., 2014. Diversity of prokaryotic community at a shallow marine hydrothermal site elucidated by Illumina Sequencing Technology: Curr Microbiol, 69, 457-466. https://doi:10.1007/s00284-014-0609-5
Lizarralde, D., Axen, G. J., Brown, H. E., Fletcher, J. M., González‐Fernández, A., Harding, A. J., Holbrook, W.S., Kent, G.M., Paramo, P., Sutherland, F., Umhoefer, P.J., 2007, Variable styles of rifting in the Gulf of California: Nature, 448, 466–469, https:// doi .org /10 .1038 /nature06035
Lloyd, K.G., Schreiber, L., Petersen, D.G., Kjeldsen, K.U., Lever, M.A., Steen, A.D., Stepanauskas, R., Richter, M., Kleindienst, S., Lenk, S., Schramm, A., Jorgensen, B. B., 2013, Predominant archaea in marine sediments degrade detrital proteins: Nature, 496, 215–218. https://doi.org/10.1038/nature12033
MacLeod, F., Kindler, G.S., Wong, H.L., Chen, R., Burns, B.P., 2019, Asgard archaea: Diversity, function, and evolutionary implications in a range of microbiomes: Microbiology, 5, 48-61. https://doi.org/10.3934/microbiol.2019.1.48
Madigan, M.T., Martinko, J.M., Parker, J., 2003, Brock biology of microorganisms, 10th ed., Upper Saddle River, Prentice Hall-Pearson Education Inc., 1012 p.
Maugeri, T.L., Gugliandolo, C., Caccamo, D., Stackebrandt, E., 2001, A polyphasic taxonomic study of thermophilic bacilli from shallow, marine vents: Systematic and Applied Microbiology, 24(4), 451–468. https://doi.org/10.1078/0723-2020-00054
Maugeri, T.L., Gugliandolo, C., Caccamo, D., Stackebrandt, E., 2002, Three novel halotolerant and thermophilic Geobacillus strains from shallow marine vents: Systematic and Applied Microbiology, 25, 450–455. https://doi.org/10.1078/0723-2020-00119
Maugeri, T.L., Lentini, V., Gugliandolo, C., Italiano, F., Cousin, S., Stackebrandt, E., 2009, Bacterial and archaeal populations at two shallow hydrothermal vents off Panarea Island (Eolian Islands, Italy): Extremophiles, 13, 199–212. https://doi.org/10.1007/s00792-008-0210-6
Maugeri, T.L., Bianconi, G., Canganella, F., Danovaro, R., Gugliandolo, C., Italiano, F., Lentini, V., Manini, E., Nicolaus, B., 2010, Shallow hydrothermal vents in the southern Tyrrhenian Sea: Chemistry and Ecology, 26, 285–298. https://doi.org/10.1080/02757541003693250
Meng, J., Xu, J., Qin, D., He, Y., Xiao, X., Wang, F., 2014, Genetic and functional properties of uncultivated mcg archaea assessed by metagenome and gene expression analyses: The ISME Journal, 8, 650–659. https://doi.org/10.1038%2Fismej.2013.174
Na, H., Lever, M. A., Kjeldsen, K. U., Schulz, F., Jorgensen., B. B., 2015, Uncultured desulfobacteraceae and crenarchaeotal group c3 incorporate 13c-acetate in coastal marine sediment: Environmental Microbiology Reports, 7, 614-22. https://doi.org/10.1111/1758-2229.12296
Neumann, F., Negrete-Aranda, R., Harris, R. N., Contreras, J., Sclater, J. G., González-Fernández, A., 2017, Systematic heat flow measurements across the Wagner Basin, northern Gulf of California: Earth and Planetary Science Letters, 479, 340–353. https://doi.org/10.1016/j.epsl.2017.09.037
Nobu, M.K., Narihiro, T., Kuroda, K., Mei, R., Liu, W. T., 2016, Chasing the elusive Euryarchaeota class WSA2: genomes reveal a uniquely fastidious methyl-reducing methanogen: The ISME Journal, 10(10), 2478-2487. https://doi.org/10.1038/ismej.2016.33
Nunoura, T., Nishikawa, M., Hirai, M., Shimamura, S., Harnvoravongchai, P., Koide, O., Morono, Y., Fukui, T., Inagaki, F., Miyazaki, J., Takaki, Y., Takai, K., 2018, Microbial diversity in sediments from the bottom of the challenger deep, the Mariana Trench: Microbes and Environments, 33, 186-194. https://doi.org/10.1264/jsme2.ME17194
Offre, P., Spang, A., Schleper, C., 2013, Archaea in biogeochemical cycles: Annual Review of Microbiology, 67, 437–457. https://doi.org/10.1128/AEM.02638
Oliver, G.C., Cario, A., Rogers, K.L., 2020, Rate and Extent of Growth of a Model Extremophile, Archaeoglobus fulgidus, Under High Hydrostatic Pressures: Front Microbiol, 11, 1023, 1-14. https://doi.org/10.3389/fmicb.2020.01023
Paduan, J.B., Zierenberg, R., Clague, D.A., Spelz, R. M., Caress, D.W., Troni, G., Thomas, H., Glessner, J., Lilley, M.D., Lorenson, T., Lupton, J., Neumann, F., Santa Rosa-del Rio, M.A., Wheat, C.G., 2018, Discovery of hydrothermal vent fields on Alarcón Rise and in southern Pescadero Basin, Gulf of California: Geochemistry, Geophysics, Geosystems, 19, 4788–4819. https://doi.org/10.1029/2018GC007771
Persaud, P., Stock, J.M., Steckler, M.S., Martín-Barajas, A., Diebold, J.B., González-Fernández, A., Mountain, G.S., 2003, Active deformation and shallow structure of the Wagner, Consag, and Delfín Basins, northern Gulf of California, Mexico: Journal of Geophysical Research, 108, 2355, 1-27. https://doi.org/10.1029/2002JB001937
Pettit, L.R., Hart, M.B., Medina-Sánchez, A.N., Smart, C.W., Rodolfo-Metalpa, R., Hall-Spencer, J.M., Prol-Ledesma, R.M., 2013, Foraminifera resilient to ocean acidification in the Gulf of California: Marine Pollution Bulletin, 73, 452-462. https://doi.org/10.1016/j.marpolbul.2013.02.011
Prieur, D., 1997, Microbiology of deep-sea hydrothermal vents: Trends in Biotechnology, 15(7), 242-244. https://doi.org/10.1016/S0167-7799(97)01052-4
Prol-Ledesma, R.M., Torres-Vera, M.A., Rodolfo-Metalpa, R., Ángeles, C., Lechuga Deveze, C., Villanueva-Estrada, R.E., Shumilin; E., Robinson, C., 2013, High heat flow and ocean acidification at a nascent rift in the northern Gulf of California: Nature Communications, 4, 1388, 1-7. https://doi.org/10.1038/ncomms2390
Pruesse, E., Peplies, J., Glockner, F. O., 2012, Sina: Accurate high-throughput multiple sequence alignment of ribosomal rna genes: Bioinformatics, 28, 1823-1829. https://doi.org/10.1093/bioinformatics/bts252
Pujalte, M.J., Lucena, T., Ruvira, M.A., Arahal, D.R., Macián, M.C., 2014, The Family Rhodobacteraceae, in Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., (eds), The Prokaryotes: Berlin, Springer, 439–512. https://doi.org/10.1007/978-3-642-30197-1_377
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., Peplies, J., Glockner, F. O., 2013, The silva ribosomal rna gene database project: improved data processing and web-based tools: Nucleic Acids Research, 41, 590-596. https://doi.org/10.1093/nar/gks1219
Quéméneur, M., Bes, M., Postec, A., Mei, N., Hamelin, J., Monnin, C., Chavagnac, V., Payri, C., Pelletier, B., Guentas-Dombrowsky, L., Gérard, M., Pisapia, C., Gérard, E., Ménez, B., Ollivier, B., Erauso, G., 2014, Spatial distribution of microbial communities in the shallow submarine alkaline hydrothermal field of the Prony Bay, New Caledonia: Environ Microbiol Reports, 6, 665-674. https://doi.org/10.1111/1758-2229.12184
Rajasabapathy, R., Mohandass, C., Bettencourt, R., Colaço, A., Goulart, J., Meena, R.M., 2018, Bacterial diversity at a shallow-water hydrothermal vent (Espalamaca) in Azores Island: Current Science, 115, 1-12, http://dx.doi.org/10.18520/cs/v115/i11/2110-2121
Rinke, C., Chuvochina, M., Mussig, A.J., Chaumeil, P.A., Davín, A.A., Waite, D.W., Whitman, W.B., Parks, D.H., Hugenholtz, P., 2021, A standardized archaeal taxonomy for the Genome Taxonomy Database: Nature Microbiology, 6, 946-959. https://doi.org/10.1038/s41564-021-00918-8
Rizzo, C., Arcadi, E., Calogero, R., Sciutteri, V., Consoli, P., Esposito, V., Canese, S., Andaloro, F., Romeo, T., 2022, Ecological and biotechnological relevance of Mediterranean hydrothermal vent systems: Minerals, 12, 251, 1-39. https://doi.org/10.3390/min12020251
Rosenberg, E., DeLong, E. F., Lory, S., Stackebrandt, E., Thompson, F., 2013, The prokaryotes: Berlin, Springer, 662 p. https://doi.org/10.1007/978-3-642-30141-4
Satomi, M., 2014, The Family Shewanellaceae. in, Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., (eds.), The Prokaryotes: Berlin, Springer, 597-625. https://doi.org/10.1007/978-3-642-38922-1_226
Schloss, P. D., Westcott, S. L., Ryabin, T., Hall, J. R., Hartmann, M., Hollister, E.B., Lesniewski, R.A., Oakley, B.B., Parks, D.H., Robinson, C.J., Sahl, J.W., Stres, B., Thallinger, G. G., Horn, D.J. V., Weber, C.F., 2009, Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities: Applied and Environmental Microbiology, 75, 7537–7541. https://doi.org/10.1128/AEM.01541-09
Segerer, A., Neuner, A., Kristjansson, J.K., Stetter, K.O., 1986, Acidianus infernus gen. nov., sp. nov., and Acidianus brierleyi comb. nov.: Facultatively aerobic, extremely acidophilic thermophilic sulfur-metabolizing archaebacteria: International Journal of Systematic Bacteriology, 36, 559-564. https://doi.org/10.1099/00207713-36-4-559
Shivaji, S., Srinivas, T.N.R., Reddy, G.S.N., 2014, The Family Planococcaceae, in Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., (eds.), The Prokaryotes: Berlin, Springer, 303-351. https://doi.org/10.1007/978-3-642-30120-9_351
Sievert, S.M., Ziebis, W., Kuever, J., Sahm, K., 2000, Relative abundance of Archaea and Bacteria along a thermal gradient of a shallow-water hydrothermal vent quantified by rRNA slot-blot hybridization: Microbiology, 146, 1287–1293. https://doi.org/10.1099/00221287-146-6-1287
Solden, L., Lloyd, K., Wrighton, K., 2016, The bright side of microbial dark matter: lessons learned from the uncultivated majority: Current opinion in microbiology, 31, 217-226. https://doi.org/10.1016/j.mib.2016.04.020
Spang, A., Saw, J. H., Jørgensen, S. L., Zaremba-Niedzwiedzka, K., Martijn, J., Lind, A.E., Ettema, T. J., 2015, Complex archaea that bridge the gap between prokaryotes and eukaryotes: Nature, 521(7551), 173-179. https://doi.org/10.1038/nature14447
Spang, A., Caceres, E.F., Ettema, R.J., 2017, Genomic exploration of the diversity, ecology and evolution of the archaeal domain of life: Science, 357, 6351, 1-10. https://doi.org/10.1126/science.aaf3883
Speth, D.R., Yu, F. B., Connon, S.A., Lim, S., Magyar, J.S., Peña-Salinas, M.E., Quake, S.R., Orphan, V.J., 2022, Microbial communities of Auka hydrothermal sediments shed light on vent biogeography and the evolutionary history of thermophily: The ISME Journal, 16, 1750-1764. https://doi.org/10.1038/s41396-022-01222-x
Staden, R., Judge, D.P., Bonfield, J.K., 2003, Analysing Sequences Using the Staden Package and EMBOSS, in Krawetz, S.A. and Womble, D.D., Introduction to Bioinformatics. A Theoretical and Practical Approach: Totawa, Human Press Inc., 393–410. https://doi.org/10.1007/978-1-59259-335-4_24
Stetter, K.O., 1988, Archaeoglobus fulgidus gen. nov., sp. nov.: A New Taxon of Extremely Thermophilic Archaebacteria: Systematic and Applied Microbiology, 10, 172–173. http://dx.doi.org/10.1016/S0723-2020(88)80032-8
Sun, Q.L., Wang, M.Q., Sun, L., 2015, Characteristics of the cultivable bacteria from sediments associated with two deep-sea hydrothermal vents in Okinawa Trough: World Journal of Microbiology and Biotechnology, 31, 2025-2037. http://dx.doi.org/10.1007/s11274-015-1953-8
Takai, K., Horikoshi. K., 1999, Genetic diversity of archaea in deep-sea hydrothermal vent environments: Genetics, 152, 1285–1297. https://doi.org/10.1093/genetics/152.4.1285
Takai, K., Nakamura, K., 2011, Archaeal diversity and community development in deep-sea hydrothermal vents: Current Opinion in Microbiology, 14, 282–291. https://doi.org/10.1016/j.mib.2011.04.013
Tamura, K., Stecher, G., Peterson, D., Filipski, A., Kumar, S., 2013, MEGA6: Molecular Evolutionary Genetics Analysis version 6.0: Molecular Biology and Evolution, 30, 2725–2729. https://doi.org/10.1093/molbev/mst197
Tarasov, V.G., Gebruk, A.V., Mironov, A.N., Moskalev, L.I., 2005, Deep-sea and shallow-water hydrothermal vent communities: Two different phenomena? Chemical Geology, 224, 5–39. https://doi.org/10.1016/j.chemgeo.2005.07.021
Teske, A. P., Salman, V., 2014, The Prokaryotes: Gammaproteobacteria, in Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., (eds), The Prokaryotes: Berlin, Springer, 93-134.
Teske, A., Sorensen, K.B., 2008, Uncultured archaea in deep marine subsurface sediments: have we caught them all?: The ISME Journal, 2, 3–18. https://doi.org/10.1038/ismej.2007.90
Teske, A., Hinrichs, K.U., Edgcomb, V., de Vera Gómez, A., Kysela, D., Sylva, S. P., Sogin, M. L., Jannasch, H.W., 2002, Microbial diversity of hydrothermal sediments in the Guaymas basin: evidence for anaerobic methanotrophic communities: Applied and Environmental Microbiology, 68, 1994–2007. https://doi.org/10.1128%2FAEM.68.4.1994-2007.2002
Thiel, V., Peckmann, J., Seifert, R., Wehrung, P., Reitner, J., Michaelis, W., 1999, Highly isotopically depleted isoprenoids: molecular markers for ancient methane venting: Geochimica et Cosmochimica Acta, 63, 3959-3966. https://doi.org/10.1016/S0016-7037(99)00177-5
Thompson, J.D., Higgins, D.G., Gibson, T.J., 1994, Clustal w: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice: Nucleic Acids Research, 22, 4673–4680. https://doi.org/10.1093/nar/22.22.4673
Wright, E. S., Yilmaz, L. S., Noguera, D. R., 2012, DECIPHER, a search-based approach to chimera identification for 16s rRNA sequences: Applied and Environmental Microbiology, 78(3), 717–725. https://doi.org/10.1128/aem.06516-11
Zaremba-Niedzwiedzka, K., Caceres, E.F., Saw, J.H., Bäckström, D., Juzokaite, L., Vancaester, E., Seitz, K.W., Anantharaman, K., Starnawski, P., Kjeldsen, K.U., Stott, M.B., Nunoura, T., Banfield, J.F., Schramm, A., Baker, B.J., Spang, A., Etterma, T.J.G., 2017, Asgard archaea illuminate the origin of eukaryotic cellular complexity: Nature, 541, 7637, 353-371. https://doi.org/10.1038/nature21031
Zhou, M., Dong, B., Liu, Q., 2016, Draft genome sequence of Psychrobacter piscatorii strain LQ58, a psychrotolerant bacterium isolated from a deep-sea hydrothermal vent: Genome announcements, 4, e00044-16. https://doi.org/10.1128/genomea.00044-16
Zhou, Z., St. John, E., Anantharaman, K., Reysenbach, A.L., 2022, Global patterns of diversity and metabolism of microbial communities in deep-sea hydrothermal vent deposits: Microbiome, 10, 241, https://doi.org/10.1186/s40168-022-01424-7
Peer Reviewing under the responsibility of Universidad Nacional Autónoma de México.
This is an open access article under the CC BY-NC-SA license(https://creativecommons.org/licenses/by-nc-sa/4.0/)

