|
Boletín de la Sociedad Geológica Mexicana Volumen 75, núm. 2, A220323, 2023 http://dx.doi.org/10.18268/BSGM2023v75n2a220323
|
 |
Fossil relatives of extant parasitic crustaceans from the Mesozoic of Europe
Parientes fósiles de parásitos actuales de crustáceos, del Mesozoico de Europa
Mario Schädel1,*, Christina Nagler2, Matúš Hyžný3
1 Institute of Evolution and Ecology, University of Tübingen, Auf der Morgenstelle 28, 72076 Tübingen, Germany.
2 Zoomorphology group, Department of Biology II, Ludwig-Maximilians-Universität München, Großhaderner Straße 2, 82152 Planegg-Martinsried, Germany.
3 Department of Geology and Paleontology, Comenius University in Bratislava, Mlynská dolina, Ilkovičova 6, 842 15 Bratislava, Slovakia.
* Corresponding author: (M. Schädel) This email address is being protected from spambots. You need JavaScript enabled to view it.
How to cite this article:
Schädel, M., Nagler, C., Hyžný, M., 2023, Fossil relatives of extant parasitic crustaceans from the Mesozoic of Europe: Boletín de la Sociedad Geológica Mexicana, 75 (2), A220323. http://dx.doi.org/10.18268/BSGM2023v75n2a220323
Manuscript received: January 1, 2023; Corrected manuscript received: March 16, 2023; Manuscript accepted: March 21, 2023.
ABSTRACT
The fossil record of Isopoda includes remains of presumed parasites. Among the fossils which have been discussed as potential parasites are those termed as Urda Münster, 1840. Some of these fossils have been discussed as possibly related to an extant group of parasites, Gnathiidae Leach, 1814. The type species of Urda – Urda rostrata Münster, 1840 – is herein interpreted as a close relative of the group Gnathiidae, based on the shared occurrence of a number of apomorphic features. This is with Urda punctata (Münster, 1842) herein being interpreted as a junior subjective synonym of U. rostrata. However, not all of the fossils associated with the name Urda can safely be identified as close relatives of Gnathiidae. Moreover, it is unclear whether the extinct species, which can be identified as close relatives of U. rostrata and Gnathiidae form a monophyletic group, as we could not identify an autapomorphy for a natural group Urda. A new species of close relatives of Urda rostrata and Gnathiidae – Urda buechneri n. sp. – is formally described based on µCT image data. Palaega suevica Reiff, 1936 and Palaega kessleri Reiff, 1936 are found to be subjective synonyms and are re-interpreted as Urda suevica n. comb. – a species closely related to U. rostrata. Due to the documented destruction of the holotype, a herein figured fossil specimen is designated as the neotype of Urda suevica. Palaega? stemmerbergensis Malzahn, 1968 is also interpreted as a close representative of U. rostrata and herein treated as Urda stemmerbergensis n. comb. Another already formally described species – Eobooralana rhodanica gen. et comb. nov. – is interpreted as a more distant relative, which is likely to be closer related to other extant species of Isopoda than those within Gnathiidae. For three species there are not enough characters preserved to interpret them as closely related to U. rostrata and Gnathiidae: Urda? liasica Frentzen, 1937 nom. dub. (type material destroyed, description insufficient for proper diagnosis), Urda? moravica Remeš, 1912 and Urda? zelandica Buckeridge and Johns, 1996.
Keywords: Isopoda, Urda, Gnathiidae, parasitism, mouthparts.
RESUMEN
El registro fósil de Isopoda incluye restos de posibles parásitos. Entre los fósiles que han sido discutidos como parásitos potenciales se encuentra Urda Münster, 1840. Algunos de estos fósiles han sido discutidos como posibles parientes de un grupo existente de parásitos, los Gnathiidae Leach, 1814. La especie tipo de Urda – Urda rostrata Münster, 1840 – es aquí interpretada como pariente cercano del grupo Gnathiidae, con base en la presencia común de un número de caracteres apomórficos. Esto incluye a Urda punctata (Münster, 1842) interpretada aquí como sinónimo junior subjetivo de U. rostrata. Sin embargo, no todos los fósiles asociados con el nombre Urda pueden ser indudablemente identificados como parientes cercanos a Gnathiidae. De manera adicional, no es aún claro si las expecies extintas, que podrían ser identificadas como cercanas a U. rostrata y Gnathiidae, forman un grupo monofilético, dado que no podemos identificar alguna autapomorfía para un grupo natural Urda. Una nueva especie de parientes cercanos a Urda rostrata y Gnathiidae – Urda buechneri n. sp. – es descrita formalmente con base en datos de imágenes µCT. Palaega suevica Reiff, 1936 y Palaega kessleri Reiff, 1936 son interpretados como sinónimos subjetivos y reinterpretados como Urda suevica n. comb. – como especies cercanamente relacionadas a U. rostrata. Debido a la documentada destrucción del holotipo, un ejemplar fósil aquí ilustrado, es designado como el neotipo de Urda suevica. Palaega? stemmerbergensis Malzahn, 1968 es también interpretada como como pariente cercano a U. rostrata y es tratada aquí como Urda stemmerbergensis n. comb. Otra especie ya descrita formalmente – Eobooralana rhodanica gen. et comb. nov. – es interpretada como un pariente más distante, quien probablemente se encuentra relacionada a otra especie viviente de Isopoda, que con los Gnathiidae. No existen caracteres suficientes preservados para tres especies, a fin de interpretarlas como cercanamente relacionadas a U. rostrata and Gnathiidae: Urda? liasica Frentzen, 1937 nom. dub. (material tipo destruído, descripción insuficiente para una adecuada diagnosis), Urda? moravica Remeš, 1912 y Urda? zelandica Buckeridge y Johns, 1996.
Palabras clave: Isopoda, Urda, Gnathiidae, parasitismo, partes bucales.
- Introduction
Isopoda is a morphologically diverse and species-rich group of eucrustaceans (Brandt and Poore, 2003). Most widely known to the general public by its terrestrial forms – ‘woodlice’ – many lineages of Isopoda have representatives that live in aquatic habitats, which is also assumed for the earliest representatives of Isopoda (e.g. Lins et al., 2012). The feeding modes within Isopoda vary extremely between its different ingroups. There are highly specialised herbivores (e.g., wood boring species of the group Limnorioidea) (Daniel et al., 1991), generalists, predators, parasites and even hyperparasites (parasites of parasites) (e.g. Rybakov, 1990). Parasites within Isopoda come from a number of different groups; how closely these groups are related to each other or if they form a monophyletic group is still a matter of ongoing research (Brusca and Wilson, 1991; Dreyer and Wägele, 2001; Brandt and Poore, 2003; Nagler et al., 2017). Hosts of these parasites are either fishes (Chondrichthyes and Actinopterygii) (e.g. Abd El-Atti, 2020) or different kinds of aquatic crustaceans such as shrimps, crabs, barnacles and other representatives of Isopoda (e.g. An et al., 2015). There is a substantial variation in the degree of dependence between the parasites and their hosts, ranging from ectoparasites that hide in reefs when not feeding (Brandt and Poore, 2001) to endoparasites that drastically reduce the sclerotization of their exoskeletons once entered the host (e.g. Shiino, 1954) but are thought to be closely related to each other if not forming a monophyletic group. Overall, compared to other ingroups of Eucrustacea, remains of representatives of Isopoda are rather rare in the fossil record (cf. Luque et al., 2017). Nevertheless, in some deposits fossil remains of Isopoda can be frequent (Walther, 1904; Haack, 1933). The oldest fossils of Isopoda are from the Pennsylvanian (Schram, 1970, 1974), with an almost continuous record during the Mesozoic and the Cenozoic (Wieder and Feldmann, 1992; Feldmann et al., 2008; Hyžný et al., 2013; Schädel et al., 2020). Although many fossil representatives of Isopoda are quite similar in their overall appearance, the fossil record of Isopoda covers a wide range of body shapes and sizes (Wieder and Feldmann, 1989; Polz, 1998; Serrano-Sánchez et al., 2016). The fossil record of Isopoda also comprises species for which a parasitic lifestyle can be assumed based on their phylogenetic position and on morphological features of the body, such as claws and mouth cones that would allow the animal to cling to a host and suck body fluids from it (Schädel et al., 2019; van der Wal et al., 2021)
Fossils associated with the genus name Urda Münster, 1840, in contrast to most other representatives of Isopoda, seemingly lack extant analogues with a similar body shape and similar morphological features (Taylor, 1972). The first finding of such fossils is from the lithographic limestones of the Solnhofen area in Southern Germany (Münster, 1840, p. 184, 1842; Kunth, 1870). These fossils are strongly compressed and there is not much brightness- or colour-contrast between preserved cuticle and the sediment. For a long time, it was not clear how many trunk segments there are in the type species of Urda – Urda rostrata Münster 1840 – and its relatives (Münster, 1840; Ammon, 1882; Stolley, 1910). This and the lack of well-preserved mouthparts and locomotory legs have led to disparate assumptions regarding the phylogenetic position and the feeding mode of U. rostrata and related species (Ammon, 1882; Carter, 1889; Monod, 1926; Menzies, 1962). Studies on well-preserved fossils (Feldmann et al., 1994; Nagler et al., 2017) showed that the number of trunk segments is the same as in the ground pattern of Isopoda and in representatives of most of its ingroups (Wägele, 1989). Nagler et al. (2017) studied multiple well-preserved fossil specimens of Urda from the Middle Jurassic of Germany with the aid of microcomputer tomography (µCT). This has revealed many aspects of the morphology and allowed for a much more detailed comparison to extant representatives of Isopoda.
In this study we compare fossils of the type species of Urda, i.e., Urda rostrata, to other fossils that have been attributed to Urda, with the goal to find autapomorphies for a group Urda and to identify which fossils actually can be attributed to the group based on apomorphic character states. By this we also re-examine the µCT scans from Nagler et al. (2017). Our new findings are discussed with regard to their implications on the functional morphology and the phylogenetic relationship of the fossils within Isopoda.
- Material and methods
2.1. MATERIAL
The fossil and extant specimens presented in this study come from multiple collections, including those of museums and universities as well as those of private collectors. The fossils originate from Mesozoic sediments of Central Europe and Great Britain.
2.2. INSTITUTIONAL ABBREVIATIONS
AM – Australian Museum, Sydney, Australia.
CeNak – Centre for Natural History, Hamburg, Germany.
ES – Natural History Museum, Bielefeld (NaMU), Germany.
GPIT – University of Tübingen, geological collection, Tübingen, Germany.
GSE – British Geological Survey, Edinburgh, UK.
JME – Jura Museum Eichstätt, Eichstätt, Bavaria, Germany.
KG – British Antarctic Survey, Station KG, Fossil Bluff, Alexander Island.
PIMUZ – Palaeontological Institute and Museum of the University of Zurich, Switzerland.
SMNK – State Museum of Natural History, Karlsruhe, Germany.
SNSB – BSPG – Bavarian State Collection for Palaeontology and Geology (part of the Bavarian Natural History Collections), Munich, Germany.
SM – Sedgwick Museum of Earth Sciences (University of Cambridge), Cambridge, UK.
2.3. DATA SOURCES
Three µCT data sets were obtained from MorphDBase (Grobe and Vogt, 2009). They are available under creative commons licences at https://www.morphdbase.de/?C_Nagler_20170221-M-130.1 (SNSB – BSPG 2011 I 50, permalink) and at https://www.morphdbase.de/?C_Nagler_20170221-M-131.1 (SNSB – BSPG 2011 I 51, permalink) along with the publication of Nagler et al. (2017). One µCT data set is reused from Nagler and Haug (2016) and is now available in the Zenodo online repository at https://doi.org/10.5281/zenodo.7010104
Information about the correlation of (bio-) stratigraphic units was retrieved from Hopson et al. (2008), Owen (2002), from the databank of the Sedgwick Museum of Earth Sciences, University of Cambridge http://www.3d-fossils.ac.uk/fossilType.cfm?typSampleId=20003067 (accessed 22.03.2021), and from Ogg et al. (2016). Numerical ages are according to Ogg et al. (2016).
2.4. IMAGING
Images of the fossils were recorded using different macro photography setups including a Canon Rebel T3i DSLR camera in combination with a Canon EF 18-55 mm f/3.5-5.6 objective and a Canon MP-E 65 mm f/2.8 1-5x objective and a Nikon D7200 DSLR camera in combination with a Laowa 100mm f/2.8 2x objective. Additionally, microscopic images were recorded using a Keyence VHX 6000 digital microscope and a Keyence BZ 9000 digital fluorescence microscope. For the digital fluorescence microscope an emitting light source with a mean wavelength of 360 nm and a band width of 40 nm (used for DAPI stains) and an emitting light source with a mean wavelength of 470 nm and a band width of 40 nm (used for GFP stains) were used (Haug et al., 2011; Eklund et al., 2018). To obtain fluorescence images with the macro photography setup, a 10 W TATTU U2S ultraviolet light torch with a ZWB2 filter (emitting light of 365 nm wavelength) was used in combination with a UV light filter mounted on the camera objective (e.g. Tischlinger and Arratia, 2013). For one specimen fluorescence was induced by equipping white-light sources with cyan filters and the image was captured using a red filter mounted onto the camera objective (“green-orange fluorescence” Haug et al., 2009; Haug and Haug, 2011). Where possible, diffuse lighting conditions (e.g., using flash diffusers) or cross-polarised light (Bengtson, 2000; Kerp and Bomfleur, 2011) was used to obtain images with fewer reflections. Some objects were imaged using an EPSON Perfection 1640SU flatbed scanner. The objects were placed in different left-right positions onto the surface of the scanner to obtain images from different viewing angles (Schubert, 2000; Haug et al., 2013).
X-ray computer tomography (µCT) was performed at the Zoological State Collection in Munich using a Baker Hughes (General Electrics) ‘phoenix nanotom m’ computer tomograph with a wolfram target on a cvd diamond, along with the acquisition software ‘datos|x’ (provided by the manufacturer). All objects scanned for this study were rotated 360 degrees in steps of 0.25 degrees, resulting in total scan times of 48 minutes for each object. The scans were performed with the following x-ray source settings: 120 kV, 100 µA. The volumetric data were computed with the software VGStudio MAX 2.2.6.80630 (Volume Graphics, proprietary). The resulting voxel sizes of the volumetric data are 4.55246 µm for the specimen from Reiff (1936, ‘Fundstück F’, GPIT-PV-76948), 13.86661 µm for ES/jb – 8744 and 18.44640 µm for the specimen pair ES/jb – 30755 and ES/jb – 30756 (preserved in the same rock, scanned together). The volumetric data are available in TIF format from the Zenodo online repository under the following links: GPIT-PV-76948 (https://doi.org/10.5281/zenodo.7010162); ES/jb – 8744 (https://doi.org/10.5281/zenodo.7011283) and ES/jb – 30755 (https://doi.org/10.5281/zenodo.7010968).
2.5. IMAGE PROCESSING
Images of different focal planes were fused (‘extended depth of field’) (Pieper and Korpel, 1983; Itoh et al., 1989) using either CombineZP/CZBatch (Alan Hadley, GPL) in combination with WINE (for running Windows applications on Linux, LGPL) or enfuse (GPL) in combination with Hugin (image alignment, GPL v.2.0). In some cases, the blue colour channel was removed using ImageMagick (Apache 2.0 license) prior to the focus merging to eliminate glow effects around highly fluorescent particles in the final images. Example scripts for the use of the command line tools are available at https://github.com/mcranium/merfoc (personal repository of the first author). Panoramic stitching was performed either manually using the unified transform tool and layer masks in GIMP v.2.10.14 (GPL v.3.0) or automatically using the ‘Grid/ Collection stitching’ plugin (Preibisch et al., 2009, GPL v.2.0) for ImageJ (public domain).
The red-cyan stereo anaglyph images included in this publication were either obtained as such (creative commons license) or created manually from images of slightly different viewing angles (Wheatstone, 1838; Rollmann, 1853) using GIMP. Red-cyan stereo anaglyph images can be converted to other formats such as paired stereo images or to wiggle images using free software such as GIMP or kataglyph (GPL v.3.0, available at https://github.com/mcranium/kataglyph).
For images from microscopy setups with fixed magnifications, scale bars were created from known pixel lengths, using ImageJ (public domain). In some cases, enfuse or MacroFusion (graphical interface for enfuse, GPL) were used to combine the dynamic range of multiple images of the same view, resulting in images without under- or overexposed areas (HDR, high dynamic range) (Fraser et al., 2009). The images were optimised for colour, brightness, contrast (‘levels’ and ‘curves’) and sharpness (‘unsharp mask’) using GIMP. In some cases, uninformative background was removed (layer masks) or simulated (‘clone’ tool, marked by dotted lines and explicitly stated in the figure captions. This was also done using GIMP.
2.6. 3D RECONSTRUCTION
Volume rendering of the µCT data was performed using Drishti 2.6.5 (MIT licence) (Limaye, 2012). Additionally, biological structures in 2D slices of the µCT data were labelled manually using TrakEM2 (Cardona et al., 2012) in Fiji (GPL v.2.0) (Schindelin et al., 2012). In one case Biomedisa (Lösel et al., 2020) was used to compute interspersed labels based on the available image data. The label maps were processed using the ‘joint’, ‘gaussian’ and ‘median’ smoothing algorithms in 3DSlicer (BSD style license) (Fedorov et al., 2012; Kikinis et al., 2014) and subsequently exported as 3D meshes. Some of the meshes were post-processed with the decimate, subdivision surface and remesh modifiers in Blender 2.8.3 (GPL v.2.0) (e.g. Sutton et al., 2014). Two-dimensional images were rendered using the ‘Cycles’ raytracing engine and a combination of ‘sun’ and ‘world’ lighting in Blender.
2.7. DATA VISUALISATION AND GRAPHIC DESIGN
The visualisation of the age of the fossils and their geographical distribution were created using R v.4.04 (GPL v.2) and the packages dplyr (Wickham et al., 2020), reshape2 (Wickham, 2007), ggplot2 (Wickham, 2009), ggtext (Wilke, 2020), deeptime (Gearty, 2021), sf (Pebesma, 2018), rnaturalearth (South, 2017) and tmap (Tennekes, 2018). The visualisation of the ages parallels a ‘Gantt chart’ (Gantt, 1910). The drawings and the arrangement of the figure plates and labels were done in Inkscape v.1.0.1 (GPL v.3.0).
2.8. BODY ORGANISATION AND TERMINOLOGY WITHIN ISOPODA
The body of most representatives of Isopoda is composed of one ocular segment and 19 post-ocular segments (PO 1–19). It consists of a head (PO 1–6) and a trunk (PO 7–19). The trunk is divided into an anterior part (pereon, PO 7–13) with walking/grasping appendages, a posterior part (pleon PO 14–18) with swimming/ventilation appendages (pleopods) and the last trunk segment that is conjoined with the telson (pleotelson, PO 19) and has swimming/steering appendages (uropods).
In some representatives of Isopoda, such as in adults of Gnathiidae, postocular segment 7 is functionally incorporated into the head. The anterior-most appendages of the head are the antennula (PO1) and the antenna (PO2). The subsequent appendages form the mouthparts: mandible, maxillula, maxilla and maxilliped. In many representatives of Isopoda there is a complex of three structures anterior or antero-dorsal to the mouthpart appendages: frontal lamina, clypeus and labrum (from anterior to posterior). In representatives of Gnathiidae the frontal lamina is not developed as a distinct structure and the labrum is either not developed (Monod, 1926; Wilson et al., 2011) or conjoined with the clypeus. The clypeus or a conjoined structure, consisting of clypeus and labrum, functionally forms an ‘upper lip’. Posterior to the mandible but arising from the same segment there is a pair of sternal lobes (paragnaths) that are functionally part of the mouthparts.
The legs of postocular segments 7–13 consist of 7 elements, each: coxa, basipod, ischium, merus, carpus, propodus and dactylus (from proximal to distal). In representatives of Scutocoxifera (ingroup of Isopoda) the coxae of the anterior trunk are conjoined with the lateral parts of the tergite and form a scale-like sclerite lateral to the rest of the tergite (coxal plate) (Dreyer and Wägele, 2002). In many representatives of Scutocoxifera the coxal plate of postocular segment 7 is conjoined with the rest of the tergite. In larval forms of Gnathiidae and Urda it is not clear whether there is a coxal plate in postocular segment 7. In the larval forms of Gnathiidae the coxa (or the coxal plate) of this segment is separated from the tergite (or from the rest of the tergite) and in the adult forms the coxa is (as is the tergite) conjoined with the head capsule. In Gnathiidae the first leg of the anterior is functionally part of the mouthparts. In Gnathiidae this leg (PO7) is often referred to as ‘gnathopod’ (larval forms) and ‘pylopod’ (adults).
- Results
3.1. UPPER JURASSIC REMAINS FROM THE SOLNHOFEN AREA – TYPE MATERIAL OF URDA ELONGATA MÜNSTER, 1840
Material: 1 specimen, complete body, SNSB BSPG AS 493, holotype of Urda elongata Münster, 1840, figured in Münster, 1840, pl. 1 fig. 3, lower Tithonian, Hybonoticeras hybonotum Zone, Solnhofen, Bavaria, Germany.
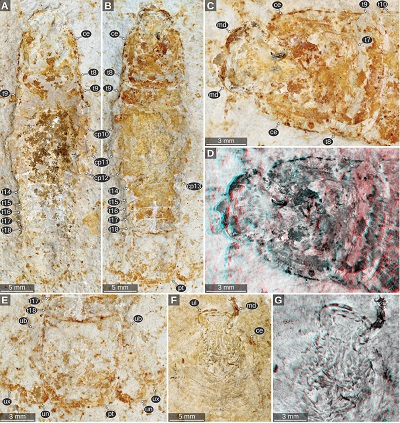
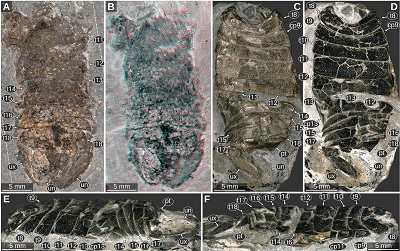
Important morphological features: Total body length 43 mm, body slender (Figures 1B, 1C). Eyes large, extending to the posterior margin of the head (Figures 1B, 1D). Upper lip large, with rounded antero-lateral corners (Figure 1D). Mandibular incisor large, projected in anterior direction, curved 90 degrees inwards, distal part of the incisor slender and with a pointed tip, distal parts of the left and right incisor extensively overlapping (Figures 1B, 1D). Pleon tergite 3 slightly narrower than pleon tergite 2, posterior margin with distinct convex mid part (Figure 1E). Pleotelson posterior margin straight. Uropod endopod extending to the level of the posterior margin of the pleotelson. Uropod exopod narrower and shorter than the endopod (Figures 1B, 1C).
Remarks: In this specimen there is no indication of a long antenna or antennula, as it was drawn in Münster (1840, pl. 1 fig. 3). In contrast to the drawings in Kunth (1870, pl. 18 figs. 1–2, depicting a different specimen of the same species), the mandibles do not appear to be forked and the upper lip extends much more in anterior direction (Figure 1D).
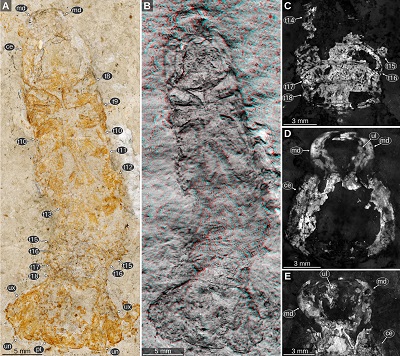 |
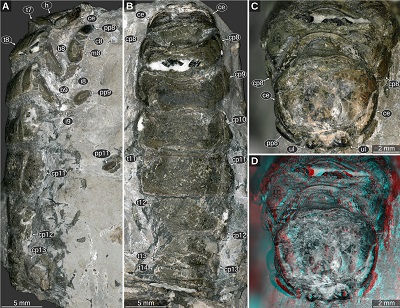
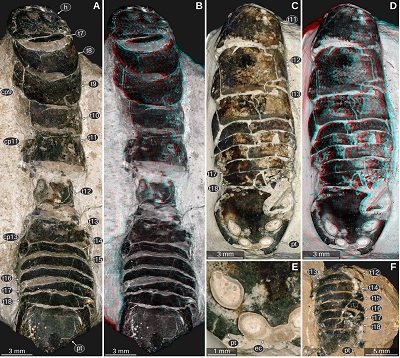
| Figure 1. Urda rostrata Münster, 1840. A–D: SNSB BSPG AS 493 syntype of ‘Urda elongata’ (Münster 1840 pl. 1 fig. 3), Upper Jurassic, lower Tithonian, Hybonoticeras hybonotum Zone, Solnhofen, Bavaria, Germany. A: white light microscopy. B: red-cyan stereo anaglyph. C: pleon region, epifluorescence microscopy. D: head region, epifluorescence microscopy. E: SNSB BSPG AS 496 syntype of ‘Reckur punctatus’ (Münster 1842 pl. 10 fig. 10; Kunth 1870 pl. 18 figs. 3, 3a), Upper Jurassic, lower Tithonian, Hybonoticeras hybonotum Zone, Daiting, Bavaria, Germany, anterior region of the head, epifluorescence microscopy. ce, compound eye; md, mandible; pt, pleotelson; t8–18, tergites of post-ocular segments 8–18; ub, uropod basipod; ul, upper lip; un, uropod endopod; ux, uropod exopod. |
3.2. UPPER JURASSIC REMAINS FROM THE SOLNHOFEN AREA – TYPE MATERIAL OF RECKUR PUNCTATUS MÜNSTER, 1842
Material: 1 specimen (part and counterpart), holotype of Urda punctata (Reckur punctatus) Münster, 1842, SNSB BSPG AS 496 and MB.A.0921 (part and counterparts are in different museums), figured in Münster, 1842, pl. 4 fig. 10 as ‘Reckur punctatus’ and in Kunth, 1870, pl. 18 figs. 3, 3a as ‘Urda punctata’ (clearly depicting MB.A.0921), Upper Jurassic, lower Tithonian, Hybonoticeras hybonotum Zone, Daiting, Bavaria, Germany.
Important morphological features: Total body length 52 mm. Eyes large, extending to the posterior margin of the head (Figure 2). Upper lip large. Mandibular incisor large, projected in anterior direction, curved 90 degrees inwards (Figure 1A). Pleon tergite 3 posterior margin with distinct convex mid part (Figure 2A). Pleotelson posterior margin straight to slightly concave (Figure 2B).
Remarks: The upper lip in this specimen is not well preserved and the structures that are interpreted by Kunth (1870, pl. 18 fig. 3) as the anterior and lateral margins could also be parts of the mandibles (Figure 1A). A triangular structure on the ventral side of the head, as depicted in Kunth (1870, pl. 18 fig. 3a) corresponds to a gap between the proximal parts of the mandibular incisor, the sclerite in this place is not delimited posteriorly and likely corresponds to the dorsal part of the head capsule (the fossil appears to be accessible in ventral view).
 |
| Figure 2. Urda rostrata Münster, 1840 (Urda punctata sensu Kunth 1870), SNSB BSPG AS 496 syntype of ‘Reckur punctatus’ (Münster, 1842 pl. 4 fig. 10; Kunth, 1870 pl. 18 figs. 3, 3a), Upper Jurassic, lower Tithonian, Hybonoticeras hybonotum Zone, Daiting, Bavaria, Germany. A: epifluorescence microscopy. B: white light microscopy. C: red cyan stereo anaglyph. ce, compound eye; cp13, coxal plate of post-ocular segment 13; md, mandible; pt, pleotelson; t14–18, tergites of post-ocular segments 14–18; ub, uropod basipod; ux, uropod exopod. |
3.3. UPPER JURASSIC REMAINS FROM THE SOLNHOFEN AREA – ADDITIONAL MATERIAL
Material: 11 specimens figured herein, many of them complete bodies in various qualities of preservation, Upper Jurassic, lower Tithonian, Hybonoticeras hybonotum Zone or lacking further information, from the Solnhofen/Eichstätt area, Bavaria, Germany. Not figured but inspected are: 6 specimens of the Redenbacher collection (MB.A.922a-b – MB.A.927), 1 specimen of the Edinger collection (MB.A.4219) and 1 specimen figured in Kunth (1870, pl. 18 fig. 1–2, MB.A.920).
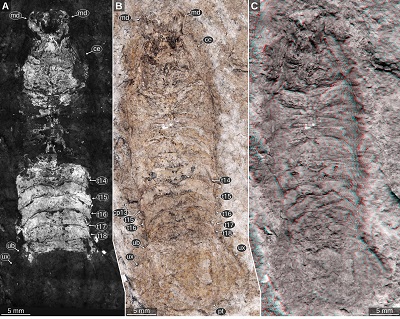
Important morphological features: Total body lengths (complete specimens only): 36.6 mm (Figure 3C), 39 mm (Figure 4A), 42 mm (Figure 5B), 44.4 (Figure 6A), 60–67 mm (Figure 7, specimen slightly distorted). Body slender, widest in the mid-part at the level of post-ocular segments 10–11 (Figures 3, 4A, 5A, 5B). Eyes large and elongate, extending to the posterior margin of the head, consisting of at least 5 rows of ommatidia, slightly tapering towards the posterior end (Figures 4D, 5C, 5D). Upper lip with proximal joint straight and wide, distal part wider than proximal part, latero-distal corners rounded (Figure 6). Antennula or antenna elements longer than wide (Figure 6D). Mandibles sturdy, with longitudinal edges (Figures 5F–5G). Tergite of PO7 short and narrower than the head, with distinct convex posterior margin (Figures 3D, 7A, 7B). Pleon with lateral outline straight and about parallel, slightly tapering towards the posterior end. Pleon tergites 1–3 with posterior margin overall concave, convex in the mid-part and concave in the lateral parts (Figures 3B, 3C). Pleotelson on the ventral side with transverse rounded ridges in the anterior half, from the lateral sides of the anterior margin to the mid-part of the lateral margin (Figure 3C). Pleotelson posterior margin straight to slightly concave in the mid-part (Figure 6).
 |
| Figure 3. Urda rostrata Münster, 1840, private collection of ‘Leptolepides’ (German private collector), Upper Jurassic, lower Tithonian. A–C: specimen 1, Schernfeld (Eichstätt), Bavaria, Germany. A: red-cyan stereo anaglyph. B–C: UV light (365 nm) macro photography. B: specimen 1 C: specimen 1, counterpart to A and B. D: specimen 2, Blumenberg (Eichstätt), Bavaria, Germany, UV light (365 nm) macro photography, composite image of part and counterpart. ce, compound eye; cp 12–13, coxal plates of post-ocular segments 12–13; md, mandible; pt, pleotelson; t7–18, tergites of post-ocular segments 7–18; ub, uropod basipod; ul, upper lip; un, uropod endopod; ux, uropod exopod. |
 |
| Figure 4. Urda rostrata Münster, 1840, macro photography, images are courtesies of the collectors. A: private collection of Herbert Gratt (Brixlegg, Austria), Upper Jurassic, lower Tithonian, Hybonoticeras hybonotum Zone, Wegscheid (Eichstätt), Bavaria, Germany. B: private collection of Manfred Ehrlich (Böhl-Iggelheim, Germany), Upper Jurassic, lower Tithonian, Blumenberg, Eichstätt, Bavaria, Germany. C: private collection of Udo Resch (Eichstätt, Germany), Upper Jurassic, lower Tithonian, Schernfeld (Eichstätt), Bavaria, Germany. D: private collection of Manfred Ehrlich (Böhl-Iggelheim, Germany), Upper Jurassic, lower Tithonian, Blumenberg, Eichstätt, Bavaria, Germany. E: private collection of Falk Starke (Bodenwerder, Germany), Upper Jurassic, lower Tithonian, Schernfeld, Bavaria, Germany. cc, calcite crystals, ce, compound eyes; cp10–13, coxal plates of post-ocular segments 10–13; h, head; md, mandible; t7–18, tergites of post-ocular segments 7–18; pt, pleotelson; ul?, possible remain of the upper lip; un, uropod endopod; ux, uropod exopod. |
 |
| Figure 5. Urda rostrata Münster, 1840, macro photography. A–E: JMS-288, private collection of Udo Resch (Solnhofen), Upper Jurassic, lower Tithonian, Blumenberg (Eichstätt), Bavaria, Germany. B: counterpart of A. C: counterpart of A, head region. D: same view as C, red-cyan stereo anaglyph. E: counterpart of A, pleotelson region. F–G: JME SOS 1794, lower Tithonian, Upper Jurassic, greater Solnhofen area, Bavaria, Germany. G: red-cyan stereo anaglyph. ce, compound eye; cp 10–13, coxal plates of post-ocular segments 10–13; md, mandible; pt, pleotelson; t7–18, tergites of post-ocular segments 7–18; ub, uropod basipod; ul, upper lip; un, uropod endopod; ux, uropod exopod. |
 |
| Figure 6. Urda rostrata Münster, 1840, private collection of Daniel Fauser (Schwäbisch Gmünd, Germany), Upper Jurassic, lower Tithonian, Wegscheid (Eichstätt), Bavaria, Germany. Note the preservation of the pleotelson and the uropod endopod. A–B: macro photography, diffused white light illumination. B: detail of the head region. C–D: UV light (365 nm) macro photography. D: detail of the head region. an, element of either antennula or antenna; an?, possible remain of either antennula or antenna; atu, antennula; ce, compound eye; cp10, coxal plate of post-ocular segment 10; md, mandible; pt, pleotelson; t7–18, tergites of post-ocular segments 7–18; ul, upper lip; un, uropod endopod. |
 |
| Figure 7. Urda rostrata Münster, 1840, private collection of Norbert Winkler (Stahnsdorf, Germany), Upper Jurassic, lower Tithonian, Hybonoticeras hybonotum Zone, Wegscheid (Eichstätt), Bavaria, Germany, green-orange fluorescence macro photography, desaturated. A: positive side. B: negative side. C–D: details of the head and anterior-most trunk region, red-cyan stereo anaglyphs based on luminescence-inverted fluorescence images. C: positive side. D: negative side. E: composite image of the positive and the negative side with focus on the fluorescent body parts. a11, appendage of post-ocular segment 11; a12?, possible appendage of post-ocular segment 12; b8–11, basipods of post-ocular segments 8–11; ce, compound eye; cp9–13, coxal plates of post-ocular segments 9–13; i8, ischium of post-ocular segment 8; m8, merus of post-ocular segment 8; md, mandible; pt, pleotelson; t7–18, tergites of post-ocular segments 7–18; ul, upper lip; ux, uropod exopod. |
3.4. LOWER CRETACEOUS FOSSIL REMAINS FROM CAMBRIDGE, UK
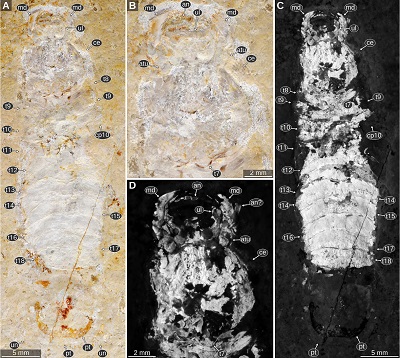
Material: 3 specimens, syntypes of Urda mccoyi (Palaega McCoyi) (Carter, 1889), partially preserved bodies including head, trunk and pleotelson, SM B 23295, SM B 23296, and SM B 23297, figured in Carter (1889, pl. 6 figs. 1–2, 4–7) as ‘Palaega McCoyi’ and in Feldmann, Wieder and Rolfe (1994, fig. 2.3–2.4, 2.6) as ‘Urda mccoyi’, Lower Cretaceous, Albian, Cambridge, Cambridgeshire, England, UK.
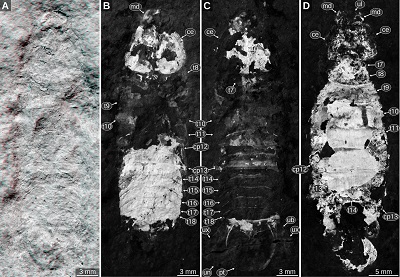
Important morphological features: Total body length about 30 mm (reconstructed from Figure 8A, 8C, 8E). Body elongate, with about parallel lateral outlines. Head roughly rectangular in dorsal view, posterior side of the head straight. Eyes on the lateral sides of the head, with posterior end at about two thirds of the length of the head. Tergite of PO7 very short, narrower than the head, posterior side convex. Tergite of PO8 much longer than that of PO7 and wider than the head. Coxal plates of PO8–9 with straight lateral margin parallel to the lateral margin of the tergite (Figures 8A, 8B). Coxal plate of PO10 anterior part wide, posterior part narrower. Coxal plates of PO11–13 anterior part narrow, posterior part wider. Tergite of PO13 postero-lateral corner pointed or tightly rounded (Figures 8C, 8D). Pleon tergites with lateral parts curved ventrally. Pleon tergites 3–4 with posterior margins evenly concave. Pleotelson gradually tapering towards the posterior side, posterior-most part not preserved in the syntypes (Figures 8E, 8F).
 |
| Figure 8. Urda mccoyi (Carter, 1889) sensu Feldmann, Wieder, and Rolfe (1994), syntypes, lower Cretaceous, Albian, Cambridge, Cambridgeshire, England, UK, images from 3d-fossils.ac.uk (CC BY-NC-SA 3.0). A–B: SM B 23295, dorsal view. A: macro photography. B: red-cyan stereo anaglyph. C–D: SM B 23296, dorsal view. C: macro photography. D: red-cyan stereo anaglyph. E–F: SM B 23297, dorsal view. E: macro photography. F: red-cyan stereo anaglyph. ce, compound eye; cp8–13, coxal plates of post-ocular segments 8–13; h, head; pt, pleotelson; t7–16, tergites of post-ocular segments 7–16. |
3.5. LOWER CRETACEOUS REMAINS FROM ALGERMISSEN, GERMANY
Material: 3 specimens, syntypes of Urda cretacea Stolley, 1910, one of them almost complete, two partially preserved, all of them no longer available (destroyed in a museum fire), results based on the detailed description and the figures Stolley (1910, pl. 6 figs. 2–4) as, Lower Cretaceous, Aptian, ‘middle Gault’, ‘Acanthoplites Schichten’, Algermissen (Hildesheim), Lower Saxony, Germany.
Important morphological features: Total body length about 50 mm. Head rectangular in dorsal view, anterior margin with a straight median portion (proximal joint of the upper lip) and paired concave rounded incisions lateral to it (space for the proximal elements of the antennula). Eyes large and elongate, posterior end at about two thirds of the length of the head. Upper lip large, elongate bulge along the midline, anterior margin with a rounded median process. Tergite of PO7 very short, narrower than the head. Subsequent tergites of the anterior trunk much longer than that of PO7. Coxal plate of PO8 triangular. Coxal plate of PO9 parallelogram shaped in lateral view. Coxal plates of PO11–12 large, with straight lateral sides parallel to the lateral margins of the tergites, antero-lateral corner angled, postero-lateral corner rounded. Pleon tergites with straight posterior margins, lateral parts curved to to ventral side. Pleon tergites 2–5 with pointed postero-lateral corners. Pleotelson about as wide as long, lateral margins in the anterior part curved to the ventral side, posterior margin evenly rounded.
Remarks: In the original description Stolley (1910) listed only 6 tergites of the anterior trunk, as opposed to 7 (PO7–13) in the ground pattern of Isopoda. However, in one of the original photographs (Stolley, 1910, pl. 6 fig. 2) a very short and wide structure is visible between the head and the subsequent tergite, most likely corresponding to the tergite of PO7.
3.6. MIDDLE JURASSIC REMAINS FROM THE CHŘIBY MOUNTAINS, CZECH REPUBLIC
Material: 1 specimen, partially preserved (posterior body region), collection of the University of Vienna, specimen not accessed; results based on the description and the figures illustrated in Remeš (1912, pl. 1 figs. 1–3) as ‘Urda moravica’, Middle Jurassic, Bathonian, ‘Braunjura epsilon’, Chřiby mountain region, near Koryčany, Zlín Region, Czech Republic.
Important morphological features: Body elongate, much longer than wide. Length of preserved body parts (PO11?–pleotelson) 23 mm. Segments of the anterior trunk long. Pleon segments much shorter than the segments of the anterior trunk, posterior margins about straight, with slightly convex mid part and concave lateral parts, based on the drawing (Remeš, 1912, pl. 1 fig. 4). Pleotelson longer than wide, posterior margin with narrow straight mid part.
Remarks: Remeš (1912) interpreted the fossil to represent the complete body of the animal. However, what Remeš (1912) interpreted as the head, likely corresponds to the fourth or the fifth segment of the anterior trunk, the eyes being coxal plates and the large mandibles being the lateral margins of the trunk segment.
3.7. MIDDLE JURASSIC REMAINS FROM AUBENAS, FRANCE
Material: 1 specimen, holotype of Urda rhodanica (Van Straelen, 1928), partially preserved (posterior body region, PO9–pleotelson), Institut de Géologie de I’Université de Lyon, Callovian, Aubenas, Ardèche, France. Specimen not accessed, results based on the description and the figures (Van Straelen, 1928, p. 13, text fig. 1, pl. 1 fig. 1).
Important morphological features: Body large, about 90–100 mm (estimation by Van Straelen 1928), longer than wide. Coxal plates of PO9–13 with transverse furrow in the anterior part. Coxal plates of 10–11 of about the same size; coxal plates of PO11–13 increasing in size. Pleon segment 2 narrower than pleon segment 1. Pleotelson about as long as coxal plate of PO13, in the anterior part with an elevation orthogonal to the midline, with a carina along the midline posterior to the elevation, posterior margin concave in the median part. Uropod endopod and exopod distally extending up to the level of the pleotelson posterior margin.
3.8. LOWER JURASSIC REMAINS FROM REUTLINGEN, GÖPPINGEN AND AALEN, GERMANY
Material: 1 specimen, paratype of Palaega kessleri Reiff, 1936, figured in Reiff (1936, ‘Fundstück A’, fig. 1a–c, pl. 1 figs. 4–5), GPIT-PV-76947, Lower Jurassic, Pliensbachian, ‘Lias delta’, Amaltheenton Formation, Reutlingen, Baden-Württemberg, Germany. 1 specimen, paratype of Palaega kessleri Reiff, 1936, figured in Reiff (1936, ‘Fundstück B’, fig. 2), collection of the municipal museum of Natural History in Göppingen, without accession number, Lower Jurassic, Pliensbachian, ‘Lias delta’, Amaltheenton Formation, Holzheim (Göppingen), Baden-Württemberg, Germany. 2 specimens, holotype of Palaega kessleri Reiff, 1936, figured in Reiff (1936, ‘Fundstück C’, figs. 3–4, pl. 1 figs. 1–3, pl. 2 figs. 1–2), paratype of Palaega kessleri Reiff, 1936, figured in Reiff (1936, ‘Fundstück D’, fig. 5), collection of the State Museum of Natural History Karlsruhe, destroyed during World War II (E. Frey, 2020, pers. comm.), Lower Jurassic, Pliensbachian, ‘Lias delta’, Amaltheenton Formation, Reichenbach (Aalen), Baden-Württemberg, Germany.
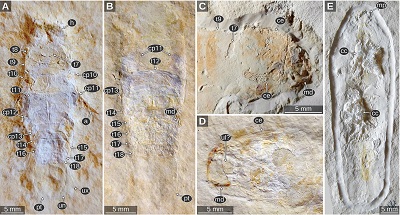
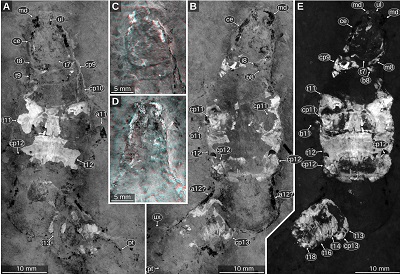
Important morphological features: Total body length roughly 30 mm (Figures 9C, 9D). Body elongate, widest at trunk segment 5. Head widest in the posterior part, anterior margin with a straight median portion (proximal joint of the upper lip) and paired concave rounded incisions lateral to it (space for the proximal elements of the antennula). Eyes large, elongate, posterior end extending to the posterior margin of the head, dorsal margin straight, ventral shorter than the dorsal margin (Figure 9A). Prominent dorsoventral ridge on the lateral side of the head directly anterior to the eyes (Figures 10A, 10B). Upper lip large, along the midline with slight elongate bulge, anterior margin with a rounded median process (Reiff, 1936, fig. 3b). Antennula with proximal-most element about as wide as long and with a flat surface parallel to the dorsal surface of the head (Figures 10A, 10B). Tergite of PO7 very short, barely visible in the photograph, not depicted in the original drawings (Reiff, 1936 pl. 2 figs 1–2). PO8 with distinct concave anterior margin (Figures 9C, 9D, 9F). PO11–13 longer than the preceding segments (Figures 9C–F, 10A, 10B). Uropod exopod narrow, distal end acute with a rounded tip (Figures 9C, 9D).
 |
| Figure 9. Urda suevica (Reiff, 1936) n. comb. A–B: syntype of ‘Palaega kessleri ’ (Reiff 1936, fig. 2, ‘Fundstück B’), Natural History Museum Göppingen, without accession number, Lower Jurassic, Pliensbachian, Göppingen, Germany. A: cross-polarised light microscopy, areas left and right to dotted lines are added digitally. B: macrophotography, red-cyan stereo anaglyph. C–F: syntype of ‘Palaega kessleri ’ (Reiff 1936, fig. 1, pl. 1, fig 4–5, ‘Fundstück A’), GPIT-PV-76947, Lower Jurassic, Pliensbachian, Reutlingen, Germany. C: dorsal view, white light microscopy, HDR. D: dorsal view, cross-polarised light microscopy. E: lateral view from the left body side, cross-polarised light microscopy. F: lateral view from the right body side. cp9–13, coxal plates of post-ocular segments 9–13; pt, pleotelson; t8–13, tergites of post-ocular segments 8–13; ub, uropod basipod; un, uropod endopod; ux, uropod exopod. |
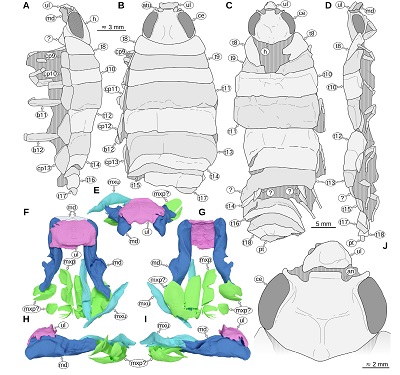 |
| Figure 10. Urda suevica (Reiff, 1936) n. comb. A–B: syntype of ‘Palaega kessleri ’ (Reiff, 1936, fig. 3, pl. 1 figs 1–3, pl. 2 figs 1–2, ‘Fundstück C’), SMNK, object destroyed, Early Jurassic, Pliensbachian, Reichenbach (Aalen), Germany. A: lateral view from the left body side, redrawn from Reiff (1936, fig. 3c). B: dorsal view, redrawn from Reiff (1936, fig. 3a). C–D, J: syntype of ‘Palaega suevica’ (Reiff, 1936, fig. 7, pl. 1 figs. 6–9, pl. 2 fig. 3, ‘Fundstück E’), SMNK, object destroyed, Lower Jurassic, Pliensbachian, Holzheim (Göppingen), Germany, redrawn from Reiff (1936). C: dorsal view, redrawn from Reiff (1936, fig. 7a). D: lateral view from the right body side, redrawn from Reiff (1936, fig. 7c). E–I: syntype of ‘Palaega suevica’ (Reiff 1936, fig. 10, pl. 3, fig. 4-6, ‘Fundstück F’), GPIT-PV-76948, Lower Jurassic, Pliensbachian, Kirchheim unter Teck, Germany, 3D models based on µCT scanning data. E: frontal view. F: dorsal view, light blue area with dotted outline depicts broken-off parts that are visible in the original figures (Reiff 1936). G: ventral view. H: lateral view from the left body side. I: lateral view from the right body side. J: same specimen as in C–D, detail of the head in dorsal view, redrawn from Reiff (1936, fig. 8a). an, antennular notch; atu, antennula; b11–12, basipods of post-ocular segments 11–12; ce, compound eye; cp9–13, coxal plates of post-ocular segments 9–13; h, head; md, mandible; mxp, maxilliped; mxp?, possibly part of the maxilliped; mxu, maxillula; pt, pleotelson; t8–18, tergites of post-ocular segments 2–7; ul, upper lip; ?, unknown body part. |
3.9. LOWER JURASSIC REMAINS FROM GÖPPINGEN AND KIRCHEIM UNTER TECK, GERMANY
Material: 1 specimen, holotype of Palaega suevica Reiff, 1936, figured in Reiff (1936, ‘Fundstück E’, figs. 7–9, pl. 1 figs. 6–9, pl. 2 fig. 3), collection of the State Museum of Natural History Karlsruhe, destroyed during World War II (E. Frey, 2020, pers. comm.), Lower Jurassic, Pliensbachian, ‘Lias delta’, Amaltheenton Formation, Holzheim (Göppingen), Baden-Württemberg, Germany. 1 specimen, paratype of Palaega suevica Reiff, 1936, figured in Reiff (1936, ‘Fundstück F’, fig. 10, pl. 2 fig. 4–6) as ‘Palaega suevica’, GPIT-PV-76948, Lower Jurassic, Pliensbachian, ‘Lias delta’, Amaltheenton Formation, Kirchheim unter Teck, Baden-Württemberg, Germany.
Important morphological features: Total body length roughly 55 mm (Figures 10C, 10D). Body elongate, widest at PO11. Head widest in the posterior part, anterior margin with a straight median portion (proximal joint of the upper lip) and paired concave rounded incisions lateral to it (space for the proximal elements of the antennula). Eyes large, elongate, posterior end extending to the posterior margin of the head, dorsal margin straight in lateral view, ventral margin straight and shorter than the dorsal margin, anterior margin slightly convex in lateral view, posterior margin oblique and straight in lateral view (Figures 10D, 10J, 11A, 11F). Prominent dorsoventral ridge on the lateral side of the head directly anterior to the eyes (Figures 10C, 10J). Upper lip large, along the midline with slight elongate bulge, anterior margin with a rounded median process, proximal-most part with a distinct transverse ridge on the dorsal side (Reiff, 1936, fig. 10; Figures 10E–I, 11B–F). Mandible incisor large strongly curved inwards, with a pointed tip (Reiff, 1936, figs. 7b, 8, 10, pl. 2 fig. 6), lateral side of the incisor with a longitudinal ridge (Figures 11B, 11F), ventral side of the incisor with a curved ridge (Figures 11B, 11C). Maxillula about as long as the anterior-posterior extent of the mandibles, slender, straight, tapering towards the distal end, dorsal side with a curved longitudinal ridge (Figures 10F, 10G). Maxilliped wider than the maxillula, proximal part possibly with a leaf shaped lateral expansion (Figures 10F, 10G); alternatively, this structure could be part of the head capsule. Tergite of PO7 short (Figure 11E), see also the gap along the midline between the posterior margin of the head and the anterior margin of the subsequent tergite (Figure 10C). Tergite of PO8 with distinct concave anterior margin (Reiff, 1936, fig. 5; Figure 10C).
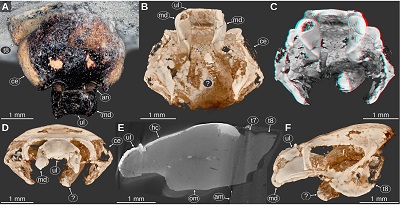 |
| Figure 11. Urda suevica (Reiff, 1936) n. comb., syntype of ‘Palaega suevica’ (Reiff 1936, fig. 10, pl. 3, fig. 4-6, ‘Fundstück F’), GPIT-PV-76948, Lower Jurassic, Pliensbachian, Kirchheim, Germany. A: dorsal view, cross-polarised light microscopy, high dynamic range. B–D, F: volume rendered images from µCT scanning data, orthographic projection. B–C: fronto-ventral view. C: red-cyan stereo anaglyph. D: frontal view. E: raw µCT volume, median-sagittal plane. F: lateral view from the rigt body side, mirrored. am, artificial matrix (likely gypsum); an, antennular notch; ce, compound eye; hc, head capsule; md, mandible; om, original sediment matrix; t7–8, tergites of post-ocular segments 7–8; ul, upper lip; ?, unknown body part or sediment structure. |
3.10. LOWER JURASSIC REMAINS FROM ÖSTRINGEN, GERMANY
Material: 1 specimen, holotype of Urda liasica Frentzen, 1937, posterior part of the body, figured in Frentzen (1937, text fig. 1b), collection of the State Museum of Natural History Karlsruhe, destroyed during World War II (E. Frey, 2020, pers. comm.), Lower Jurassic, Toarcian, Phlyseogrammoceras dispansum Zone, ‘Lias zeta’, Dinkelberg, small hill north of Östringen, Baden-Württemberg, Germany.
Important morphological features: Body elongate, length of the preserved part 15 mm (PO11–pleotelson). PO11–12 long, with large coxal plates. Pleon tergites much shorter and of about the same width than the tergites of the anterior trunk region. Pleotelson elongate, about as wide as long, with an evenly rounded posterior margin.
Remarks: From the drawing it is not completely apparent to which segments some of the sclerites belong. The presence of 3 pairs of coxal plates suggests that the anterior-most sclerite belongs to PO11.
3.11. LOWER CRETACEOUS REMAINS FROM STEMMERBERG (HANNOVER), GERMANY
Material: 1 specimen, holotype of Palaega stemmerbergensis Malzahn, 1968, massively affected by pyrite decay to the time of the original description, figured in Malzahn (1968 pl. 58, figs. 1, 2, 4–6), collection of the Niedersächsisches Landesamt für Bodenforschung, specimen lost or misplaced (C. Heunisch, 2019, pers. comm.), Lower Cretaceous, Hauterivian, Endemoceras noricum Zone, drill core ‘Stemmerberg 7’, Stemmerberg (Hannover), Lower Saxony, Germany.
Important morphological features: Body elongate, total body length about 27 mm (Malzahn, 1968, p. 828). Head wider than long (Malzahn, 1968, fig. 4), anterior margin of the head with a straight median portion (proximal joint of the upper lip) and paired concave rounded incisions lateral to it (space for the proximal elements of the antennula) (Malzahn, 1968, figs. 1–2). Eyes large, on the lateral sides of the head, elongate, kidney shaped, with pentagonal and hexagonal ommatidia (Malzahn, 1968, p. 829). Antennula with proximal article about as wide as long and with a flat to slightly convex surface parallel to the dorsal surface of the head (Malzahn, 1968, figs. 1–2). Upper lip large (Malzahn, 1968, figs. 1–2). Mandible incisor large, curved inwards (Malzahn, 1968, p. 829). Tergite of PO7 short and narrow (Malzahn, 1968, fig. 4). Leg of PO7 on the ventral side of the head and projected anteriorly (Malzahn, 1968, p. 829). PO8 with coxal plate about rectangular (Malzahn, 1968, p. 830). Pleon tergite 5 longer along the midline than preceding tergites (Malzahn, 1968, fig. 5). Pleotelson about as wide as long (Malzahn, 1968, fig. 5).
3.12. UPPER JURASSIC REMAINS FROM THE HURIWAI RIVER, NEW ZEALAND
Material: 1 specimen, holotype of Urda zelandica Buckeridge and Johns, 1996, posterior part of the body, figured in Grant-Mackie et al. (1996 figs. 3–5), A406 collection of the Geology Department, University of Auckland, Upper Jurassic, middle to upper Tithonian, locality R13/f7080, Huriwai River, near Port Waikato, North Island, New Zealand.
Important morphological features: Body elongate, length of the preserved part (trunk segment 6 to pleotelson) 15.1 mm (Grant-Mackie et al., 1996, p. 36). Pleotelson slightly wider than long, posterior margin evenly rounded.
Remarks: The description in Grant-Mackie et al. (1996) rests upon the assumption that there are only 6 tergites of the anterior trunk. Therefore, their PO11 is herein interpreted as PO12.
3.13. MIDDLE JURASSIC REMAINS FROM BIELEFELD, GERMANY – MATERIAL PRESENTED IN NAGLER et al. (2017)
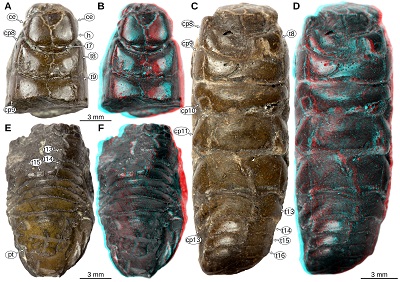
Material: 2 specimens, SNSB – BSPG 2011 I 50a,b figured in Nagler et al. (2017, fig. 1A–B, D, G, fig. 3A–C, fig. 4A6, fig. 6) as ‘Urda rostrata’ and SNSB – BSPG 2011 I 51, figured in Nagler et al. (2017, fig. 1C, E, fig. 2, fig. 3D–F, fig. 4A1–5, 7, B1–7, C1–3, fig. 5) as ‘Urda rostrata’, Middle Jurassic, Bajocian, Parkinsonia parkinsoni Zone, quarry ‘Bethel 1’, Bielefeld, North Rhine-Westphalia, Germany.
Important morphological features: Body elongate, much longer than wide, total body length about 35 mm (Figure 12A). Head anterior margin with a straight median portion (proximal joint of the upper lip) and paired shallow concave rounded incisions lateral to it (space for the proximal elements of the antennula), posterior margin straight (Figures 12A–12C, 13C, 13D). Eyes on the lateral sides of the head, elongate, posterior end at about ¾ of the heads length (Figures 12A, 13A, 13C, 13D). Lateral side of the head on the anterior end with distinct dorsal-ventral ridge (anterior to the eye) (Figures 12A–12C). Antennula proximal-most element with flat surface parallel to the dorsal surface of the head, subsequent elements about cylindrical, much narrower than the proximal-most element. Antenna short, two elongate cylindrical elements (‘peduncle’), followed by multiple much shorter elements (‘flagellum’; Figure 14). Upper lip large, wider than long, trapezoid, distal part wider than proximal part, anterior margin with a rounded median process (Figures 12B, 12C). Mandible incisor large, about 90 degrees curved inward, with a pointed tip (Figures 12B, 12C, 14C, 14D, 14G, 14H, 15K, 15L). Tergite of PO7 very short and narrower than the head, posterior margin straight (Figures 12A, 13A). Leg of PO7 parallel to the ventral side of the head, its distal end pointing in anterior direction (to the mouth parts), coxa short, not visible in lateral view, basipod widening towards the distal end, ischium about as long as the preceding element, widening towards the distal end, merus much shorter than the preceding element, carpus triangular, shorter than the preceding element, propodus large, much longer and wider than the preceding element, lateral surface convex, median surface flat, dactylus thin, gently curved inwards, about as long as the preceding element (Figures 14C, 14D, 14G, 14H, 16G, 16H). Tergite of PO8 much longer than the preceding tergite and wider, about as wide as the head (Figures 12A, 13A, 14E, 14F).
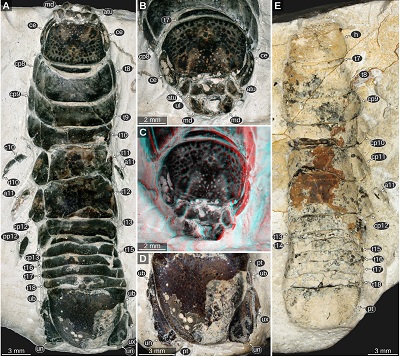 |
| Figure 12. Urda buechneri n. sp., Middle Jurassic, Bajocian, quarry ‘Bethel 1’, Bielefeld, North Rhine-Westphalia. A–D: SNSB – BSPG 2011 I 50a (figured in Nagler et al., 2017 as ‘Urda rostrata’). A: dorsal view, cross polarised light microscopy. B: head in antero-dorsal view, cross polarised light microscopy. C: red-cyan stereo anaglyph version of B. E: SNSB – BSPG 2011 I 50b (counterpart of A–D, figured in Nagler et al., 2017 as ‘Urda rostrata’), macro photography. a5, appendage of post-ocular segment 5; atu, antennula; c10, carpus of post-ocular segment 10; ce, compound eye; cp8–13, coxal plates of post-ocular segments 8–13; d10, dactylus of post-ocular segment 10; md, mandible; pp10–12, propodi of post-ocular segments 10–12; pt, pleotelson; t7–18, tergites of post-ocular segments 7–18; ub, uropod basipod; ul, upper lip; un, uropod endopod; ux, uropod exopod. |
 |
| Figure 13. Urda buechneri n. sp. SNSB – BSPG 2011 I 51 (figured in Nagler et al., 2017 as ‘Urda rostrata’), Middle Jurassic, Bajocian, quarry ‘Bethel 1’, Bielefeld, North Rhine-Westphalia, Germany, cross-polarised light microscopy. A: lateral view. B: dorsal view. C–D: head and anterior trunk region in anterodorsal view. D: red-cyan stereo anaglyph. c8, carpus of post-ocular segment 8; ce, compound eye; cp8–13, coxal plates of post-ocular segments 8–13; h, head; i8–9, ischia of post-ocular segments 8–9; m8, merus of post-ocular segment 8; pp8–11, propodi of post-ocular segments 8–11; t7–14, tergites of post-ocular segments 7–14; ul, upper lip. |
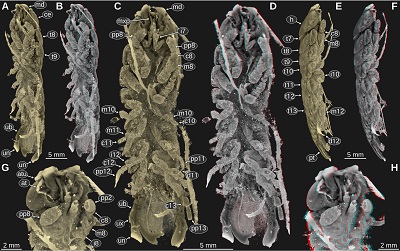 |
| Figure 14. Urda buechneri n. sp. SNSB – BSPG 2011 I 50a (figured in Nagler et al., 2017 as ‘Urda rostrata’), Middle Jurassic, Bajocian, quarry ‘Bethel 1’, Bielefeld, North Rhine-Westphalia, Germany, volume rendered images from µCT scanning data. A–B: ventro-lateral view from the left body side. B: red-cyan stereo anaglyph. C–D: ventral view. D: red-cyan stereo anaglyph. E–F: lateral view from the right body side. F: red-cyan stereo anaglyph. G–H: head and anterior trunk region in antero-ventro-lateral view from the right body side. H: red-cyan stereo anaglyph. at, antenna; atu, antennula; c8–13, carpi of post-ocular segments 8–13; ce, compound eye; d11–12, dactyli of post-ocular segments 11–12; h, head; i7–12, ischia of post-ocular segments 7–12; m8–11, meri of post-ocular segments 8–11; md, mandible; mxp, maxilliped; t7–13, tergites of post-ocular segments 7–13; pp8–13, propodi of post-ocular segments 8–13; pt, pleotelson; ub, uropod basipod; un, uropod endopod; ux, uropod exopod. |
Coxal plates of PO8–9 with straight lateral margin parallel to the lateral margins of the tergites (Figures 13A, 14E, 14F). Leg of PO8 much larger than the leg of the preceding segment, ischium proportionally shorter than in the leg of the preceding segment, merus lateral surface convex, larger than in the leg of the preceding segment, dactylus thin, curved inwards, about ⅔ of the length of the preceding leg element (Figures 14, 16A–D). Coxal plate 4 triangular, anterior portion wide, posterior portion narrow (Figures 14E, 14F). Coxal plates of PO11–13 anterior portion narrow and posterior portion wider (Figures 12A, 12E, 14E, 14F). Legs of PO11–13 ischium slenderer than in leg of PO8, merus flattened in anterior-posterior direction, lateral side straight, carpus widening in towards the distal end, proportionally longer than in leg of PO8, distal end with 2 spines on the median side, propodus slender, curved inwards, dactylus thin, curved inwards, about ½ of the length of the preceding element (Figures 14A–F, 16A, 16B, 16E, 16F). Tergite of PO13 shorter than preceding tergite, postero-lateral corner widely rounded (Figures 12A, 12E). Coxal plate of PO13 with posterolateral corner extending posterior to tergite of PO13, the posterior part being lateral to the anterior-most pleon tergites (Figure 12A). Pleon tergites 2–5 with lateral parts curved to the ventral side, postero-lateral corners pointed and distinctly projecting posteriorly (Figures 14A, 14B). Pleon tergite 5 longer along the midline than the preceding tergites (Figures 12A, 12E). Pleotelson about as wide as long, posterior margin evenly rounded (Figure 12D). Uropod endopod lateral margin with denticles (Figure 12D).
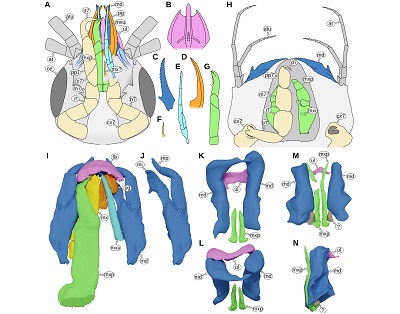 |
| Figure 15. A: Paragnathia formica (Hesse, 1864), head in ventral view, redrawn after Monod (1926, p. 75 figs. 30, 33, 34). B–G: details of A, ventral view. B: upper lip. C: mandible. D: paragnath. E: maxillula. F: possible maxilla. G: maxilliped. H: Bythognathia yucatanensis Camp, 1988, head in ventral view, redrawn from Camp (1988, pp. 670–671 figs. 1–2). I–J: Nerocila acuminata Schiödte and Meinert, 1881, 3D reconstruction based on µCT data from Nagler et al. (2017). I: mouthparts in ventral view. J: left mandible in ventral view. K–N: Urda buechneri n. sp. (Urda rostrata sensu Nagler et al. 2017), 3D reconstruction of the mouthparts based on µCT data from Nagler et al. (2017). K–L: SNSB – BSPG 2011 I 50. K: ventral view. L: antero-ventral view. M–N: SNSB – BSPG 2011 I 51, note that the distal parts of the mandibles are missing. M: ventral view. N: lateral view from the left side of the body. at, antenna; atu, antennula; b7, basipod of post-ocular segment 7; c7, carpus of post-ocular segment 7; ce, compound eye; cx7, coxa of post-ocular segment 7; d7, dactylus of post-ocular segment 7; i7, ischium of post-ocular segment 7; m7, merus of post-ocular segment 7; m7?, possible merus (and/or carpus) of post-ocular segment 7; md, mandible; mx, maxillula; mx?, possible maxillula; mxp, maxilliped; mxu, maxillula; pg, paragnath; pp7, propodus of post-ocular segment 7; ul, upper lip. |
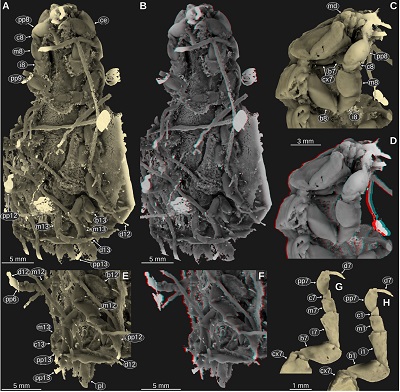 |
| Figure 16. Urda buechneri n. sp. SNSB – BSPG 2011 I 51 (figured in Nagler et al., 2017 as ‘Urda rostrata’), Middle Jurassic, Bajocian, quarry ‘Bethel 1’, Bielefeld, North Rhine-Westphalia, Germany, volume rendered images from µCT scanning data. A–B: ventral view, pleon region is missing. B: red-cyan stereo anaglyph. C–D: head and anterior trunk region in lateral view from the right body side. D: red-cyan stereo anaglyph. E–F: mid-body region in ventrolateral view from the left body side. F: red-cyan stereo anaglyph. G–H: appendage of post-ocular segment 7; G: posterior (functional lateral) view. H: anterior (functional median) view, mirrored. b7–13, basipods of post-ocular segments 7–13; c7–13, carpi of post-ocular segments 7–13; ce, compound eye; cx7, coxa of post-ocular segment 7; d7–13, dactyli of post-ocular segments 7–13; i7–8, ischia of post-ocular segments 7–8; m7–13, meri of post-ocular segments 7–13; md, mandible; pl, pleopod; pp7–13, propodi of post-ocular segments 7–13. |
3.14. MIDDLE JURASSIC REMAINS FROM BIELEFELD, GERMANY – ADDITIONAL MATERIAL
Material: 3 specimens, ES/jb-8744, ES/jb-30755, and ES/jb-30756, Middle Jurassic, Bajocian, Parkinsonia parkinsoni Zone, clay pit ‘Bethel 1’, Bielefeld, North Rhine-Westphalia, Germany.
Important morphological features: Body elongate, much longer than wide, total length about 34 mm (Figure 17A). Head anterior margin with a straight median portion (proximal joint of the upper lip) and paired concave rounded incisions lateral to it (space for the proximal elements of the antennula), posterior margin straight (Figures 17A, 17B). Eyes on the lateral side of the head, elongate, posterior end at about three quarters of the length of the head (Figures 17A, 17B). Tergite of PO7 very short and narrower than the head, posterior margin straight (Figures 17A, 17B). Tergite of PO8 much longer than the preceding tergite and wider, about as wide as the head (Figures 17A, 17B). Coxal plate of PO8 with straight lateral margin parallel to the lateral margins of the tergites. Coxal plates of PO12–13 anterior part narrow and posterior part wider (Figures 17C, 17D, 18C–F). Tergite of PO13 shorter than preceding tergite (Figures 17A–D). Coxal plate of PO13 with posterolateral corner extending posterior to tergite of PO13, the posterior part being lateral to the anterior-most pleon tergites (Figures 17A–D). Pleon tergites 2–5 with lateral parts curved to the ventral side, postero-lateral corners pointed and distinctly projecting posteriorly (Figure 18). Pleon tergite 5 longer along the midline than the preceding tergites (Figures 17A–D, 17F).
 |
| Figure 17. Urda buechneri n. sp., Middle Jurassic, Bajocian, clay pit ‘Bethel 1’, Bielefeld, North Rhine-Westphalia, Germany, cross polarised light microscopy. A–B: ES/jb-8744, dorsal view. B: red-cyan stereo anaglyph. C–E: ES/jb-30755, dorsal view. D: red-cyan stereo anaglyph. E: detail of the posterior part of the pleotelson. F: ES/jb-30756, posterior trunk region in dorsal view. cp9–13, coxal plates of post-ocular segments 9–13; ec, encrustation; h, head; pt, pleotelson; t7–18, tergites of post-ocular segments 7–18. |
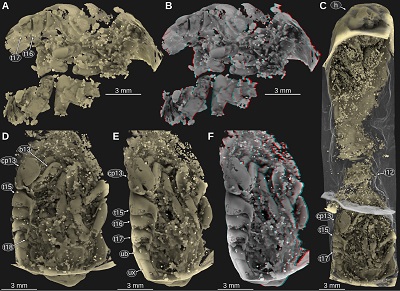 |
| Figure 18. Urda buechneri n. sp., Middle Jurassic, Bajocian, clay pit ‘Bethel 1’, Bielefeld, North Rhine-Westphalia, Germany, volume rendered images from µCT scanning data. A–B: ES/jb-30756, mid-body region in ventro-lateral view from the right body side (right side is anterior). B: red-cyan stereo anaglyph. C–F: ES/jb-8744. C: ventral view. D: pleon region in ventral view. E: pleon region in ventro-lateral view from the left body side. F: red-cyan stereo anaglyph version of E. b13, basipod of post-ocular segments 13; cp13, coxal plate of post-ocular segment 13; h, head; t12–18, tergites of post-ocular segments 12–18; ub, uropod basipod; ux, uropod exopod. |
- Discussion
4.1. THE TYPE MATERIAL OF URDA AND ADDITIONAL FOSSILS FROM SOLNHOFEN
There are numerous fossil remains of the group Urda from the lithographic limestones of the Solnhofen area in Southern Germany, which are all early Tithonian (Late Jurassic) in age. Initially, Münster (1840) described 4 species of Urda from Solnhofen, shortly afterwards Münster (1842) and Meyer (1856) described two additional species of Isopoda with a similar appearance under the generic name Reckur, which was later synonymised with Urda (Oppel, 1862; Kunth, 1870). Although it was possible to explain most of the differences between the species listed by Münster (1840, 1842) and Meyer (1856) as artefacts of preservation or negligent mistakes (e.g., the type specimen of Urda cincta is the counterpart of the type specimen of Urda decorata), Kunth (1870) did not venture to synonymise the remaining species Urda rostrata and Urda punctata, because of the morphology of the mouthparts, which seemingly differ between the species.
With the aid of fluorescence microscopy and macro photography using fluorescent light settings, we could show that the differences in the interpretation of the mouthparts (Kunth, 1870, pl. 18 figs. 1–2 vs. fig. 3) in the type material of U. elongata (= U. rostrata) (Figure 1D) and U. punctata (Figure 2A) can easily be explained by misinterpretation due to different modes of preservation. Kunth (1870) interpreted the mandible in the type specimen of U. rostrata to be bifurcate; however, in the fluorescence image (Figure 1D) it is apparent that the mandible is not bifurcate and the upper lip is much larger than depicted by Kunth (1870, pl. 18 fig. 1). Also, it is apparent from the fluorescence images that the conspicuous triangular sclerite of U. punctata depicted in Kunth (1870, pl. 18 fig. 3a) is in fact a part of the head capsule and not a distinct sclerite (Figure 2A). The upper lip morphology in the type material of U. rostrata and U. punctata is also consistent with the upper lip morphology in the herein presented additional material (Figures 5F, 5G, 6B, 6D).
There seems to be a variation in the proportional length of the anterior trunk region (cf. Figures 1 A, 5A, 5B, 7A, 7B vs. 2, 6A–C). However, it is not clear, whether this variation is due to a variation in the living animal – where it could be interpreted as a possible sexual dimorphism – or due to a post-mortem distortion. Therefore, we conclude, that there is only a single species of Urda from the lower Tithonian of Solnhofen. In this case, U. punctata is considered a junior subjective synonym of U. rostrata (see taxonomy section below).
4.2. MORPHOLOGICAL CHARACTERISTICS OF URDA
The type species of Urda – Urda rostrata Münster, 1840 – has a series of morphological features that are derived (not part of the ground pattern of Isopoda) and not present in other species of Isopoda, except for those within the group Gnathiidae Leach, 1814 (see discussion below).
The upper lip in U. rostrata is large and, despite the good preservation, neither the frontal lamina, which in other representatives of Isopoda is located dorsal to the clypeus, nor the labrum, which in other representatives of Isopoda is located ventral to the clypeus, is recognisable as a distinct structure in the fossil remains. The mandible is large, its incisor is projected towards the anterior side of the head, in dorsal view protruding from the rest of the head and strongly curved (about 90 degrees). The tergite of postocular segment 7 (the one directly posterior to the head) is very short (the subsequent tergites are much longer) and it is also not as wide as the head or the subsequent tergites.
Additional characteristics, which can also be seen be seen in other in other lineages of Isopoda, comprise the elongate shape of the body (e.g. Brandt and Poore, 2003 fig. 1A,D,G), the large eyes on the lateral sides of the head (Delaney, 1989 fig. 1C,E) and the shape of the pleotelson, lateral sides of which are about parallel in the anterior part (e.g. Camp and Heard, 1988; Bruce and Olesen, 2002 fig. 8A; Bruce, 2005; Thamban et al., 2015 fig. 8A). A concave part of the posterior margin of the pleotelson as present in some specimens of U. rostrata (Figures 2B, 6A) can also be seen in other lineages of Isopoda, such as in Aegidae (e.g. Bruce, 2009 fig. 19A,E).
4.3. REINTERPRETATION OF FOSSILS FROM THE LITERATURE (IN HISTORICAL ORDER)
Urda mccoyi (Carter, 1889) – type material only.
The type specimens of Urda mccoyi differ from U. rostrata in having considerably shorter eyes, a proportionally longer tergite of PO8 than in U. rostrata (cf. Figures 8A, 8B vs. Figures 7A, 7C) and rounded posterior margin of the pleotelson instead of a straight or slightly concave posterior margin as in U. rostrata (cf. Figures 8E, 8F vs. 1A, 5E). Additionally, the remains of U. rostrata are about 40 million years older than the type specimens of U. mccoyi.
Urda mccoyi and U. rostrata share a similar body shape. The rectangular shape of the head is also very similar, which is likely due to a similar arrangement of the mouthparts (wide upper lip joint and protruding mandibles), which is not apparent from the fossils themselves (Figures 8A, 8B). The eyes in both species are elongate and located on the lateral sides of the head (cf. Figures 8A, 8B vs. 5C, 5D). In both species, the tergite of PO7 is very short (the subsequent tergites are much longer) and narrower than the head (Figures 8A, 8B vs. 5C, 5D, 7), which is dissimilar to other representatives of Isopoda (except for those within Gnathiidae, see discussion below). Thus, it is most likely that U. mccoyi is a close relative of U. rostrata.
Urda cretacea Stolley, 1910.
The type specimens of Urda cretacea have shorter eyes than the representatives of U. rostrata. In U. cretacea the anterior margin of the upper lip has a median process (Stolley, 1910 pl. 6 fig. 4), whereas in U. rostrata the anterior margin appears to be straight or slightly convex (Figures 1D, 5F, 5G). Unlike in U. rostrata, the pleotelson in U. cretacea is evenly rounded (Stolley, 1910 pl. 6 fig. 2). In U. cretacea the head is about as wide as the tergite of PO8 and the straight portion of the posterior margin of the head in dorsal view is wide (Stolley, 1910 pl. 6 figs. 2, 4), whereas in the slightly younger (Figure 19) fossils of U. mccoyi the head is markedly narrower than the tergite of PO8 and the straight portion of the posterior margin of the head in dorsal view is narrower (Figures 8A, 8B). Additionally, the type specimens of U. cretacea are about 30 million years younger than the type specimens of U. rostrata and at least 3.6 million years younger than those of U. mccoyi.
The head morphology in U. cretacea is very similar as in Urda rostrata; in both species the upper lip is large and its proximal joint in wide and straight. Lateral to the upper lip joint, in both species there are concave incisions on the dorsal side of the head capsule, where the proximal element of the antennula is located. In both species the tergite of PO7 is very short (Stolley, 1910, pl. 6 fig. 2, not mentioned in the original description). Thus, it is most likely that U. cretacea is a close relative of U. rostrata and U. mccoyi.
Urda moravica Remeš, 1912 sensu Remeš (1912).
Although the holotype of Urda moravica resembles U. rostrata and the other two above mentioned species in some characters (elongate body, shape of the pleotelson; Remeš, 1912 fig. 1–3), similar expressions of those characters can also be found in other lineages of Isopoda as well (see discussion above). It is important to note that the interpretation in Remeš (1912) unlikely reflects the body organisation of the fossil; most notably the large mandibles described in Remeš (1912) are probably either lateral margins of a tergite or coxal plates. The allegedly present eyes are most likely coxal plates. Therefore, despite some similarities, U. moravica cannot be reliably interpreted as a close relative of U. rostrata. Furthermore, the preservation of the specimen does not allow to differentiate the species from other species such as for example Urda cretacea or the fossil remains from Bielefeld (U. rostrata sensu Nagler et al., 2017).
Urda rhodanica Van Straelen, 1928 sensu Van Straelen (1928).
Urda rhodanica can be safely identified as a species within the group Scutocoxifera based on the presence of coxal plates (Dreyer and Wägele, 2002). The head and the anterior part of the trunk are not preserved in the holotype of U. rhodanica. Consequently, it cannot be affirmed, whether the distinct morphological features that are shared between U. rostrata, U. mccoyi and U. cretacea (see discussion above) are present in representatives of U. rhodanica. Urda rhodanica also differs from the above-mentioned species in features of the posterior body part. In U. rhodanica the coxal plate of PO12 is much larger than the coxal plate of PO11 and the coxal plate of PO13 is even larger than the coxal plate of PO12, whereas in U. rostrata the coxal plate of PO13 is smaller than the preceding coxal plates (Figures 3B, 3C, 4A). The size of the coxal plates in U. rhodanica is also different from that in U. mccoyi (Figures 8C, 8D) and U. cretacea (Stolley, 1910, pl. 6 figs. 2a, 3a), the latter two species being more similar to U. rostrata in this aspect. The posterior margin of the pleotelson in U. rhodanica has a distinct concave notch, which is much more prominent than in the few specimens of U. rostrata, where the posterior margin of the pleotelson also has a concave portion (Figures 2B, 6A). Ultimately, U. rhodanica cannot be reliably interpreted as a close relative of U. rostrata. Moreover, the differences between U. rhodanica and the above-mentioned species make it also unlikely that U. rhodanica is closely related to U. rostrata.
Palaega kessleri Reiff, 1936 and Palaega suevica Reiff, 1936 sensu Reiff (1936).
Reiff (1936) noticed differences in the shape of the upper lip between specimens of Palaega kessleri (pentagonal shape, Reiff, 1936 fig. 3b, 4) and Palaega suevica (hexagonal shape, Figures 11A, 10E–G). However, the shape of the clypei only differs in the distal-most part. In the specimen ‘Fundstück C’ (P. kessleri, specimen destroyed) a transverse ridge is depicted at the place where in the specimens of P. suevica there is the distal margin. This makes it likely that the overall hexagonal upper lip shape in P. suevica is an artefact of preservation rather than an original morphological feature that distinguishes the two species.
Reiff (1936) listed a different proportional length of the pleon between Palaega kessleri (Figures 9C–F) and Palaega suevica (Figures 10C, 10D). However, this difference is probably described by the different proportional lengths of the tergites of PO10–12 (cf. Fig 10A, 10B vs. 10C, 10D). A similar variability in the lengths of these tergites can also be found in Urda rostrata (cf. Figures 6, 7) and can be well explained by sexual dimorphism (longer tergites in females due to the presence of a brood pouch). Therefore, we conclude, that the type material of Palaega kessleri and Palaega suevica originates from the same biological species. In this case, P. kessleri should be seen as the subjective synonym of P. suevica (see taxonomy section below).
The head morphology in P. suevica (incl. P. suevica in the following) is very similar to that in Urda rostrata. The upper lip is large and its proximal joint is wide and straight; lateral to the upper lip joint, there are concave incisions on the dorsal side of the head capsule (insertion point of the proximal antennula elements; Figures 11A, 10B, 10J). The mandibles are large, projected in anterior direction and strongly curved (Figures 10E–I, 11B, 11C, Reiff, 1936, pl. 2 fig. 6).
Even though not recognised by Reiff (1936), in representatives of P. suevica there is a very short tergite (PO7) visible anterior to the much longer ones of the rest of the anterior trunk region (Figure 11E, Reiff, 1936, pl. 2 figs. 1–2). Here, the morphology of the tergite of PO8 seemingly speaks against a short first tergite being present, because in PO8 the coxal plates are conjoined with the tergite (Figures 9C–F, 11A). In many representatives of Scutocoxifera, which is a monophyletic group characterised by the presence of coxal plates (Dreyer and Wägele, 2002), in PO7 the coxal plate is conjoined with the tergite. However, in larval forms of some species of Gnathiidae, where the tergite of PO7 is also very short, post-ocular segment 8 has coxal plates that are conjoined with the tergite – the morphological feature is shifted one segment posterior (Monod, 1926 fig. 13; Smit et al., 1999 fig. 31, 2003 fig. 14; Manship et al., 2011 fig. 4G). Considering the morphological features, especially those of the head, shared with U. rostrata, which are, except for representatives of Gnathiidae and the above-mentioned species, not present in other lineages of Isopoda, we interpret P. suevica as being closely related to U. rostrata.
Palaega suevica differs from U. rostrata, U. mccoyi and U. cretacea in having the coxal plate of PO8 conjoined with the tergite. Palaega suevica has a convex posterior margin of the head instead of a straight margin as in U. mccoyi and U. cretacea. The distal margin of the upper lip in U. rostrata is stout and evenly rounded (Figures 1D, 5F, 5G, 6B, 6D), whereas in P. suevica it has a distinct median convexity (Reiff, 1936, fig. 3b, 4). In addition, the remains of P. suevica are at least 30 million years older than the type material of U. rostrata and even older than the type material of U. mccoyi and U. cretacea. Therefore, it is unlikely that P. suevica is conspecific with U. rostrata or its close relatives.
Keupp and Mahlow (2017 p. 167, fig. 10) identified a fossil specimen from the Amaltheenton Formation of Buttenheim (Lower Jurassic, upper Pliensbachian, Pleuroceras spinatum Zone) as a representative of Palaega suevica sensu Reiff (1936). Being of about the same age as the specimens from Reiff (1936), the specimen in Keupp and Mahlow (2017, SNSB BSPG 2016 I 32) resembles the type specimens in having a broad straight upper lip joint and eyes that are located on the lateral sides of the head (visible in an unpublished µCT scan, Keupp and Mahlow, 2017, p. 167). Because many body parts are not exposed to the rock surface, only a detailed study of the µCT scan or further mechanical preparation will reveal further information about the possible conspecificity with the material from Reiff (1936) and the relationship to U. rostrata and the extant group Gnathiidae.
Urda liasica Frentzen, 1937 sensu Frentzen (1937).
In some respects, the holotype of Urda liasica resembles other fossils that have been associated with the genus Urda. For example, the tergites of the anterior trunk are long and the coxal plates are large; also, the pleotelson is longer than wide, its lateral margins are parallel in the anterior part and its posterior margin is evenly rounded (Frentzen, 1937 text fig. 1b). However, because only the posterior part of the body is known, the key morphological features of the type species of Urda – Urda rostrata – are not known to be present in the holotype of U. liasica. A close relationship between the type specimen of U. liasica and U. rostrata is possible, as there are no morphological features that would suggest otherwise. Yet, because the features present in the type specimen of U. liasica also occur in other lineages (see discussion above), such a close relationship cannot be inferred from the holotype.
The type material of U. liasica, consisting of a single specimen, was destroyed in World War II. Therefore, only a single drawing is available. Based on this drawing, which appears to be a rather stylised than detailed depiction, it is not possible to clearly distinguish the fossil from other fossil occurrences (cf. Figures 12A, 12D). Therefore, we suggest treating Urda liasica as a nomen dubium and its holotype as a representative of Scutocoxifera of uncertain systematic position.
Palaega stemmerbergensis Malzahn, 1968 sensu Malzahn (1968).
The holotype of Palaega stemmerbergensis shares multiple morphological features with U. rostrata, that otherwise only occur in representatives of Gnathiidae and fossil remains of close relatives of U. rostrata. The joint between the dorsal surface of the head capsule and the upper lip is wide and straight, lateral to it are concave rounded incisions, where the proximal element of the antennula is located (Malzahn, 1968 figs. 1–2). The mandible incisors are large and curved inwards (Malzahn, 1968, p. 829). The tergite of PO7 is short and narrow (Malzahn, 1968, fig. 4). The leg of PO7 is located on the ventral side of the head with its distal part pointing anteriorly (Malzahn, 1968, p. 829). The morphology of the leg of PO7 is not apparent in any of the fossils of U. rostrata from Solnhofen. However, the orientation of the first trunk leg as described by Malzahn (1968, p. 829) is very similar to that in representatives of Gnathiidae (see discussion below). Additional similarities between the type material of P. stemmerbergensis and U. rostrata that are also present in other lineages of Isopoda, comprise the elongated body shape, the position of the eyes on the lateral sides of the head (Malzahn, 1968 fig. 4, p. 829).
The holotype of P. stemmerbergensis has already been strongly deformed due to pyrite decay when it was described (Malzahn, 1968), rendering many features of the body incomparable to other specimens. Furthermore, it could not be located in the collection, where it should have been deposited (C. Heunisch, 2020, pers. comm.). This makes it impractical to differentiate P. stemmerbergensis from other species based on its morphological features. For example, the morphology of the P. stemmerbergensis type material is similar to the about 20 million years younger fossils of U. cretacea (both Early Cretaceous in age, Figure 19), yet most of the body parts where there could be differences between the type of P. stemmerbergensis and representatives of U. cretacea have not been described in detail nor are they visible in the figures of Malzahn (1968).
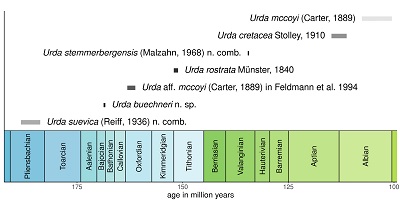 |
| Figure 19. Stratigraphic distribution of representatives of Urda Münster, 1840 (‘Gantt chart’). The depicted timespans (horizontal grey bars) do not refer to the longevity of the species but represent the possible age range of each occurrence. The grey values of the horizontal lines additionally correlate with the uncertainty of the occurrence: short dark lines for precisely and long light lines for less precisely dated occurrences. The colours of the geological scale are according to the International Chronostratigraphic Chart (v 2020/01). |
Urda zelandica Buckeridge and Johns, 1996 sensu Buckeridge and Johns (1996).
The holotype of Urda zelandica can be safely identified as a representative of the group Scutocoxifera based on the presence of coxal plates (Dreyer and Wägele, 2002). It resembles other fossils that have been associated with the name Urda in the body parts which are preserved in the specimen. Namely, this resemblance comprises the elongate body shape, the pleon tergites, which lateral parts are either stout or curved towards the ventral side (Grant-Mackie et al., 1996, figs. 3–4, p. 36), and the shape of the pleotelson, which lateral margins are about parallel in the anterior part and its posterior margin is evenly rounded or with a narrow straight mid-part (Grant-Mackie et al., 1996, fig. 5).
While the holotype of U. zelandica resembles representatives of U. rostrata in some aspects, it consists only of strongly compressed remains of the posterior body region and therefore lacks the body parts in which U. rostrata differs from other representatives of Isopoda (see discussion above). Thus, a close relationship between U. zelandica and U. rostrata cannot be reliably inferred based on morphological features. The compressed nature of the fossil and that only the posterior body region is preserved make it difficult to morphologically distinguish the type specimen of U. zelandica from other fossils and from extant representatives of Isopoda. Therefore, we suggest to treat Urda zelandica as a species of uncertain affinity within Scutocoxifera, until further material becomes available or the available material is studied using methods that allow to gather additional morphological insights.
4.3.1. The fossils from the Middle Jurassic of Bielefeld, Germany
The fossil material from Bielefeld presented in Büchner (1971) and Nagler et al. (2017) differs from the remains of Urda rostrata from Solnhofen in many aspects. In the Solnhofen material the eyes extend to the posterior end of the head (Figures 3, 4C), whereas in the material from Bielefeld the eyes end at about three quarters of the length of the head (Figures 12A, 13C, 17A). In the Solnhofen fossils the tergite of PO7 is narrow and its posterior margin is distinctly convex (Figures 3D, 7A); in the Bielefeld fossils the corresponding tergite is wider and its posterior margin is less convex (Figures 17A, 12A). The pleotelson in the Solnhofen fossils has a straight posterior margin, in some cases even with a slightly concave mid-part (Figures 1A, 1B, 5E); in the fossils from Bielefeld, however, the posterior margin is evenly rounded (Figure 12D). Additionally, the occurrence of U. rostrata from Solnhofen is about 16 million years younger than the fossils from Bielefeld (Figure 19). Therefore, it is unlikely that fossils of both localities come from a single species.
As in the fossils of U. rostrata from Solnhofen, the upper lip in the fossils from Bielefeld is also large and with a wide joint to the head capsule with rounded incision lateral to the joint, where the proximal elements of the antennula insert (Figures 12B, 12C, 13C, 13D). The mandible incisors in the Bielefeld fossils are large and strongly curved, with a pointed tip as it is the case in representatives of U. rostrata from Solnhofen (cf. Figures 15K, 15L vs. Figures 1D, 1E). The tergite of PO7 is also very short and narrower than the head and the tergite of PO8 in both the fossils from Solnhofen and the fossils from Bielefeld (Figures 17A vs. 7A). Therefore, we interpret the fossils from Bielefeld to represent a separate species, which is closely related to U. rostrata.
In the fossils from Bielefeld the legs of PO7 are preserved and their morphology, size and relative position to the head is well visible in renderings of the µCT scans (Figures 16C, 16D, 16G, 16H). The much smaller size relative to the subsequent legs and the position on the ventral side of the head, with the distal elements projected anteriorly, is very similar to the condition in larval forms of Gnathiidae (Figure 20B). A similar orientation and relative size of the legs of PO7 also occurs in some representatives of Aegidae (e.g. Nozères, 2008) and Cymothoidae (van der Wal and Haug, 2020 fig. 20).
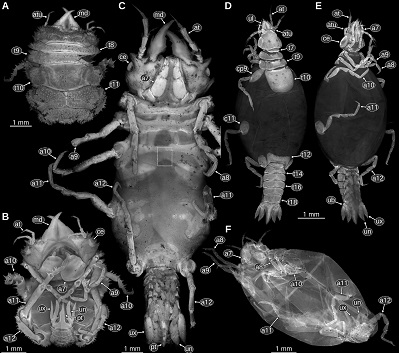 |
| Figure 20. Extant representatives of Gnathiidae, epifluorescence microscopy, 360 ± 20 nm excitation wavelength. A–B: Gnathiidae sp., adult male, CeNak K 38947-1. A: head and anterior part of the trunk in dorsal view. B: ventral view on the anterior part of the trunk, dorsal view on the pleon and pleotelson region. C: Euneognathia sp., adult male, CeNak K 40059, ventral view, dotted rectangle encompasses a digitally restored gap in the original image. D–E: Gnathia sp., praniza, CeNak K 38945-1. D: dorso-lateral view. E: ventro-lateral view. F: Gnathia sp., adult female, eggs removed from marsupium, CeNak K 38945-2, ventral view, mirrored. a7–12, appendages of post-ocular segments 7–12; at, antenna; atu, antennula; ce, compound eye; cp10–11, coxal plates of post-ocular segments10–11; md, mandible; pt, pleotelson; t7–18, tergites of post-ocular segments 7–18; ub, uropod basipod; ul, upper lip; un, uropod endopod; ux, uropod exopod. |
In the here studied remains of U. rostrata from Solnhofen the leg of PO7 is either not preserved or overlain by other structures. In µCT renders of two of the specimens from Bielefeld the maxilliped is visible (Figures 14C, 14D, 14G, 14H, 15K, 15N). The maxilliped is notably slenderer than in representatives of Cymothoidae (Figure 15I) and adult forms of Gnathiidae (Figure 15H). The slender shape of the maxilliped is similar to larval forms of Gnathiidae (e.g. Ota, 2014 fig. 13; Figure 20B). In the remains of U. rostrata from Solnhofen there is only one specimen that has a paired structure on the ventral side of the head that could potentially be remains of the maxillipeds (Figures 5F, 15G). Because of the strong similarity in the body parts that are known from both occurrences, it is likely that representatives of U. rostrata had a similar morphology of the legs of PO7 and the maxilliped as the fossils from Bielefeld.
The fossils from Bielefeld differ from representatives of U. mccoyi in having a less bulged head and a less convex posterior margin of the tergite of PO7 (cf. Figures 12B, 12C, 13A, 13B vs. 8A, 8B). Additionally, the fossil material from Bielefeld is about 60 million years older than the type fossil of U. mccoyi. From representatives of U. cretacea the Bielefeld fossils differ in having a narrower head; in U. cretacea the second tergite of the trunk is about as wide as the head (Stolley, 1910, pl. 6 figs. 2,4), whereas in the fossils from Bielefeld the second tergite of the trunk is markedly wider than head (Figures 12A, 13B). Furthermore, the fossils from Bielefeld are more than 50 million years older than the type material of U. cretacea. Representatives of P. suevica lack distinct coxal plates in PO8 (Figures 9C–F), whereas the fossils from Bielefeld clearly have distinct coxal plates in PO8 (Figures 12B, 13A). Also, in representatives of P. suevica the posterior margin of the head is convex (Figures 10B, 10C, 10J), whereas in the fossils from Bielefeld the posterior margin of the head has a straight mid-part (Figures 3B, 17A). The fossils of P. suevica are about 15 million years older than the fossils from Bielefeld. Therefore, we interpret the fossils from Bielefeld to be from a distinct species, which is closely related to U. rostrata; its description is presented in the taxonomy section below.
4.4. OTHER MENTIONS OF URDA IN THE FOSSIL RECORD
Feldmann et al. (1994) presented a single specimen (GSE 15083) from the Oxfordian (Upper Jurassic) of the Isle of Skye (UK). The specimen is complete, except for the appendages which are not preserved or not exposed to the surface of the sediment. The shape of the head is typical of U. rostrata and its close relatives, the upper lip joint is wide, there are rounded incisions where the antennula inserts and the eyes are elongate and on the lateral sides of the head. The tergite of PO7 is very short and narrower than the head (Feldmann et al., 1994 figs. 1–2, 5, 7). Therefore, and due to the overall similarity between the specimen, U. rostrata and its close relatives mentioned above, it is most likely that the fossil described by Feldmann et al. (1994) is a close relative of U. rostrata. Feldmann et al. (1994) noted the striking similarity between this specimen and the type material of U. mccoyi (Upper Cretaceous, UK), based on which they suggested that the specimen from Skye is a representative of U. mccoyi despite the age difference of at least 53 million years (Figure 19). One difference that could indicate that the specimen from the Isle of Skye might be from a different species are the dimensions of the pleotelson. In the specimen from Skye the pleotelson is wider than long (Feldmann et al., 1994, p. 89 fig. 2.7), whereas in the type material of U. mccoyi the pleotelson is more elongate (about as wide as long, Figures 8E, 8F).
From the mid-Bajocian (Middle Jurassic) of Velpe (near Osnabrück, Germany) there is one incomplete specimen (Ruhr Museum Essen, Germany), which has been associated with the genus Urda because of the shape of the pleotelson (Wittler, 2007, 2011). In this specimen only the pleon (segment 2 onwards) and the pleotelson are preserved and the specimen is lacking visible remains of appendages. This specimen is of about the same age (less than one million years older) as the fossils from Bielefeld (Büchner, 1971; Wittler, 2007). Despite the partial preservation in the fossil from Velpe, which would not allow for a robust and precise systematic interpretation of the fossil, the strong resemblance to the fossils from Bielefeld (cf. Figure 12A vs. Wittler, 2011, figs. 1–2) and the small difference in age suggest that the fossil from Velpe represents the same species as the fossils from Bielefeld.
From the lower Pliensbachian (Lower Jurassic) of Östringen (Southern Germany) there is one record of a fossil remain (SMNK, destroyed) Frentzen (1937) described as ‘Urda spec.’. The fossil consists of 3 bilateral-symmetric sclerites (Frentzen, 1937 text fig. 1a). The sclerites provide no morphological indication that they are from a representative of Isopoda (or even Eucrustacea). Also, the sclerites do not resemble those of the holotype of U. liasica (treated as a nomen dubium herein), which was found in a nearby fossil site and described by the same author (Frentzen, 1937).
There is a single fossil (PIMUZ 132a Sch 70) from the lower Aalenian (Middle Jurassic) of Schinznach-Dorf (Canton of Aargau, Switzerland), which Etter (1988) described as Urda sp. While the parts of the body that are preserved in the fossil resemble those of close relatives of U. rostrata (as discussed above), the fossil consists only of remains of the posterior region of the body (Etter, 1988 fig. 6). The similarity to U. rostrata is particularly apparent in the pleotelson which has a concave mid-part of the posterior margin, similar to some fossils of U. rostrata (Figures 2B, 6). However, similar pleotelson morphologies also occur in other lineages of Isopoda, such as in representatives of Aegidae (Bruce, 2009 fig. 19A,E). Therefore, while it is possible that the fossil from Schinznach-Dorf is a close relative of U. rostrata, there are not enough morphological characters preserved to judge this as being most likely.
From the Aptian (Lower Cretaceous) of Alexander Island (West Antarctica) there is one fossil (KG.5.16) of a representative of Isopoda, which Taylor (1972) treated as Urda cf. cretacea. Unlike interpreted in Taylor (1972), the fossil does not comprise the head and the anterior part of the trunk (Taylor, 1972, fig. 2). What has been interpreted as the head in Taylor (1972) is most likely the tergite of PO12. In the body parts that are visible in this fossil, it strongly resembles U. rostrata and its close relatives. However, none of the characteristic features of U. rostrata and its close relatives are apparent in the fossil. Therefore, despite the resemblance, there are not enough morphological characters available for a robust interpretation of the Antarctic fossil as a close relative of U. rostrata.
4.5. RELATIONSHIP BETWEEN SPECIES OF URDA AND GNATHIIDAE
The above mentioned extinct close relatives of Urda rostrata and U. rostrata itself can all be easily identified as representatives of Scutocoxifera due to the presence of coxal plates (modified parts of the coxae; Dreyer and Wägele, 2002). The pleotelsa in these species are relatively flat and the uropods are located on the ventral side of the pleotelson (their proximal joint is not lateral to the tergite of the pleotelson; Figures 1A, 1B, 14A–F). This can be interpreted as in indication that the species are representatives of the group Cymothoida (an ingroup of Scutocoxifera) (Brandt and Poore, 2003). While this character can serve as an indication, it cannot be seen as a clear autapomorphy of Cymothoida, since the polarity of this character with respect to the condition in Valvifera and Sphaeromatidea is unclear (Brandt and Poore, 2003).
Kunth (1870) interpreted Urda rostrata and its congeners, treated by him as “Urdaidae”, as intermediate forms between Gnathiidae and Cymothoidae. Urda rostrata and its extinct close relatives (recognized as congeners here) share a number of character states with the group Gnathiidae (as already noted by Van Straelen, 1928, p. 12), which are not present in other lineages of Isopoda and can therefore be seen as autapomorphies of a group that comprises Gnathiidae, U. rostrata and its close relatives (Figure 21). The anterior margin of the dorsal surface of the head has a straight median portion which is formed by the proximal joint of the upper lip and two incisions lateral to it where the proximal elements of the antennulae are located. The upper lip is large, and its proximal part is directly articulated with the head capsule; a distinct frontal lamina, as present in many lineages of Isopoda, is not developed (Monod, 1926); a distinct labrum, which in many lineages of Isopoda is located on the distal side of the clypeus is also not developed (cf. Figures 12B, 12C, 11 vs. 20A) (Wilson et al., 2011). This morphology is only present in the larval forms of Gnathiidae, as in the adult males and females the upper lip as a whole is reduced (Figure 20D; figures in Thing et al., 2015; Ota, 2019), probably due to the fact that they are no longer feeding.
The tergite of post-ocular segment 7 is very short, narrower than the head (cf. Figures 7A, 8A, 11E, 12A, 17A vs. 20A) and the legs of post-ocular segment 7 (corresponding to the first trunk legs in other representatives of Isopoda) are functionally incorporated into the head (Nagler et al., 2017). This morphology is also only present in the larval forms of Gnathiidae, because in the adults the tergite is often fully conjoined with the head capsule (Figure 20D), but sometimes a suture is visible in the adults (e.g. Manship et al., 2011, fig. 1D).
Urda rostrata and its above discussed extinct relatives can be distinguished from representatives of Gnathiidae by a series of autapomorphies of Gnathiidae (Figure 21). In adult representatives of Gnathiidae there is no well-developed leg in post-ocular segment 13 (Figures 20C, 20E, 20F) (Wilson, 1996), which seems to be a paedomorphy as in all representatives of Isopoda this appendage is not yet developed in young (manca stage) individuals (Watling, 1981; Ax, 2000, p. 176; Boyko and Wolff, 2014). On the other hand, in fossils of extinct close relatives of U. rostrata a well-developed leg in this segment is preserved (Figures 10C, 10D, 14A–D, 16A, 16B, 16E, 16F, 18C–F), which indicates that the fossils are remains of adult (or late immature) individuals that are more plesiomorphic with respect to Gnathiidae regarding this character. The absence of well-developed legs of PO13 in adults of Gnathiidae is a paedomorphic feature, as the leg is also missing in manca-stage immature individuals of all species of Isopoda and other related ingroups of the more inclusive group Mancoida.
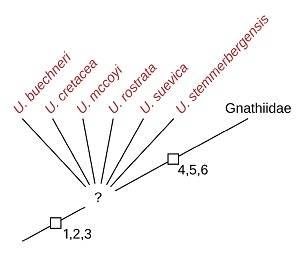 |
| Figure 21. Proposed relationship between species of Urda and the group Gnathiidae. 1, anterior margin of the head with a stra- ight median portion (proximal joint of the upper lip); 2, upper lip large, frontal lamina and labrum not developed or conjoined with other structures; 3, tergite of post-ocular segment 7 very short; 4, post-ocular segment 13 without well-developed appendages; 5, maxilliped and appendage of post-ocular segment 7 with flattened elements (adult forms); 6, mandible straight and projected anteriorly (larval forms). |
In adults of the group Gnathiidae there is an extreme sexual dimorphism and the mouthparts are not used for feeding (Wägele, 1989, fig. 93). This seems to be reflected in the morphology of the mouthparts. The appendages of post-ocular segments 6 and 7 – maxilliped and trunk leg 1 (‘pylopod’ in Gnathiidae literature) are flattened and in adults of most, but not all (Figure 15H), species of Gnathiidae the dactylus of PO7 is reduced (Cohen and Poore, 1994).
In larval forms of Gnathiidae the mandible is thin, straight and has a pointed tip (Wägele, 1989 fig. 93). In adult males of Gnathiidae the mandible is often very large and strongly curved, extending far beyond the anterior margin of the head capsule; in this, the condition in adults of Gnathiidae is more similar to the condition in U. rostrata (Monod, 1926). However, representatives of U. rostrata, P. suevica and the specimens from Bielefeld lack the blade (flat median expansion of the mandible) which is present in many males of Gnathiidae (e.g. Ota and Hirose, 2009). The shape of the mandibles in the here presented fossils is more similar to that in representatives of other lineages of Cymothoida, such as Corallanidae (Delaney, 1989 fig. 22A–B) or Protognathia (Wägele and Brandt, 1988; Kussakin and Rybakov, 1995), in which the mandibles do not extend beyond the anterior margin of the head and a well-developed labrum is present.
The fossils from Bielefeld and the fossils of U. rostrata and P. suevica, in which the mouthparts are preserved, give no indication that they are from larval or immature individuals; specifically, the legs on post-ocular segment 13 are well developed, as opposed to being not yet developed or very short as in (manca stage) immature representatives of Isopoda (Ax, 2000, p. 176). Therefore, the mandibles in immature stages of the extinct relatives of Gnathiidae could either have been similar to those of the adults (large and inwards curved; Figure 15H) or more similar to larval forms of Gnathiidae (straight or slightly outwards curved; Figure 15A, 15C).
The shape of the eyes is another character in which U. rostrata and its extinct relatives are similar to representatives of Gnathiidae, however, mostly to larval individuals of the group. In adult forms of Gnathiidae the eyes are still located on the lateral sides of the head but are much smaller compared to the size of the head than in the larvae (e.g. Ota and Hirose, 2009). Nevertheless, in some adults of Gnathiidae the eyes remain large and similar to those in the herein presented fossils (Tanaka, 2005; Ota, 2019). As there is no drastic reduction of the size of the eyes apparent in most representatives of Isopoda, the reduction of the eye size from larval to adult individuals within Gnathiidae likely represents a hypermorphosis in combination with a pre-displacement (see discussion in Haug et al., 2010), which is not shared by the extinct relatives presented herein.
The shape of the pleotelson in most species of Gnathiidae is approximately triangular, with a narrow posterior end (Figures 20A, 20B, 20E, 20F, 22E–22F). Yet there are also exceptions to that in extant species (e.g., Figure 22D) in that it is very different from that in U. rostrata and its herein presented extinct relatives, where the width of the pleotelson decreases significantly only in the posterior half and the posterior margin is either rounded or truncate (Figures 22G, 22H). Since both conditions occur in other lineages of Cymothoida as well (Bruce, 1986 fig. 35I; Messana, 2020, fig. 2), the polarity of this character, and thus the value of the pleotelson shape as a potential autapomorphy of a monophyletic group Urda, is unclear.
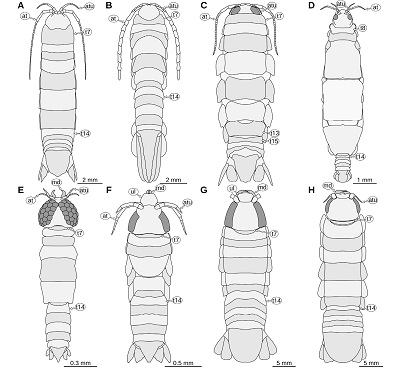 |
| Figure 22. Habitus drawings in dorsal view. A: Typhlocirolana buxtoni Racovitza, 1912, adult, redrawn from Racovitza (1912). B: Protognathia bathypelagica (Schultz, 1977), immature specimen, redrawn from Wägele and Brandt (1988). C: Corallana sp., Comprehensive Marine Biodiversity Survey, Singapore, JS-2675, drawn after a photograph by Arthur Anker, no scale available. D: Caecognathia agwillisi (Seed, 1979), adult female, redrawn from Seed (1979). E: Tenerognathia visus Tanaka, 2005, adult male, redrawn from Tanaka (2005). F: Gnathia sp., zuphea stage, Lizard Island, AM P.81399, drawn from SEM images in Wilson et al. (2011). G: Urda rostrata Münster, 1842, reconstructed from multiple fossils of the greater Solnhofen area, Germany. H: Urda buechneri n. sp., reconstructed from multiple fossils from Bethel, Germany. at, antenna; atu, antennula; md, mandible; t7–15, tergites of post-ocular segments 7–15; ul, upper lip. |
Urda rostrata and the extinct species that are herein interpreted as close relatives of it share several apomorphies with representatives of Gnathiidae but differ from them in characters that are plesiomorphic for the extinct species or of unclear polarity. This implies a close relationship between the extinct species and the extant representatives of Gnathiidae. One possibility is that the extinct species form a monophyletic group Urda. In the other case (non-monophyletic Urda), a nomenclatural dilemma arises due to the use of binomial species names. Either the name Urda is used as a name of a higher group, in which case Gnathiidae would become an ingroup of Urda, which would cause much trouble among those who care about taxonomic ranks and their reflection in the naming of groups, or alternatively the name Urda is used as the first part of the binomen Urda rostrata, in which case all species of extinct close relatives of U. rostrata need to receive a separate genus name. Because of this dilemma and because uninomial nomenclature (Lanham, 1965) is currently not accepted by the ICZN, the nomenclature herein used in the taxonomy section below is as if Urda forms a sister group to Gnathiidae, while pointing out that this is not necessarily the case. Therefore, Urdidae as a monotypic taxonomical entity ranked at the family level, proposed by Kunth, 1870, and adopted subsequently by some authors, e.g., Van Straelen, 1928; Taylor, 1972; Feldmann et al., 1994; Etter, 2014) is not followed here.
4.6. RELATIONSHIP BETWEEN URDA AND OTHER LINEAGES OF ISOPODA
Coxal plates (compound structures of the lateral parts of the tergites and the proximal leg element) are well visible in representatives of Gnathiidae and the herein discussed close relatives of the group (Figures 7, 20A); this clearly identifies them as representatives of the group Scutocoxifera (Dreyer and Wägele, 2002). Within Scutocoxifera, Gnathiidae and its extinct relatives belong to the group Flabellifera (sensu Wilson, 2003) which can be characterised by the functional grouping of the legs of the anterior trunk (legs of PO7–9 are projected anteriorly and the more posterior legs are projected posteriorly), which is not present in other representatives of Scutocoxifera, such as woodlice (Brusca and Wilson, 1991).
Within Flabellifera sensu Wilson 2003, the position of Gnathiidae, and thus also its extinct relatives, has been debated for several decades. Wägele and Brandt (1988) and Wägele (1989) assumed that Gnathiidae was more closely related to the non-parasitic forms of Cymothoida. They proposed a close relationship between the group Protognathia Wägele and Brandt, 1988 and Gnathiidae (Wägele and Brandt, 1988). However, the most important proposed synapomorphy of Protognathia and Gnathiidae, the lack of a well-developed leg on post-ocular segment 13, has later been shown to be the result of an erroneous interpretation of the holotype as an adult individual, but it is a manca stage (Kussakin and Rybakov, 1995; Wilson, 1996). In all representatives of Mancoida (of which Isopoda is an ingroup) early immature stages lack a well-developed leg on post-ocular segment 13 (Ax, 2000; Boyko and Wolff, 2014). In all species of Protognathia the tergite of PO7 is distinctly wider than the head and about as long as the subsequent tergites at least in the lateral aspect) and the leg of PO7 resembles the subsequent legs in size and orientation; also, a well-developed labrum is present (Wägele and Brandt, 1988; Kussakin and Rybakov, 1995). Therefore, it is most likely that Protognathia and Gnathiidae are less closely related than Gnathiidae and U. rostrata and all its herein discussed extinct relatives. Consequently, the slender shape the pleotelson and the uropod rami have, shared by representatives of Protognathia and most representatives of Gnathiidae (Figures 20A, 20B, 20E, 20F), has to be considered a result of convergent evolution.
Similarly, another non-parasitic species of the group Cymothoida – Gnatholana mandibularis Barnard, 1920 – has been interpreted to be a close relative of Urda rostrata and closely related extinct species (Monod, 1926, p. 639 ff.; Menzies, 1962). Representatives of Gnatholana mandibularis have large mandibles, protruding in anterior direction (well visible in dorsal view), a distinct clypeus and a distinct labrum are also projected anteriorly, similar to the upper lip in Urda rostrata. However, other aspects of the morphology in G. mandibularis are very different from representatives of U. rostrata and its extinct relatives: the head is short and wide; the eyes are not elongate; clypeus and labrum are both visible and not conjoined with each other; the tip of the mandible has 4 small teeth; the tergite of PO7 is long and much wider than the head (Barnard, 1920, p. 352 ff. pl. 15 fig. 24). The similar morphology of the mandible therefore has to be interpreted as a result of convergent evolution (cf. Brusca and Wilson, 1991, p. 167).
The group Protourda Mezzalira and Martins-Neto, 1992 has been described based on an assemblage of fossils from the Permian of the Paraná Basin (São Paulo state, Brazil). The group Protourda, according to Mezzalira and Martins-Neto (1992), comprises two species (Protourda tupiensis Mezzalira and Martins-Neto, 1992 and Protourda? circunscriptia Mezzalira and Martins-Neto, 1992). Mezzalira and Martins-Neto (1992) assumed a sister group relationship between Protourda and U. rostrata and its extinct relatives based on the shared presence of six (instead of seven) tergites of the anterior trunk. However, as shown herein, Urda rostrata has seven tergites of the anterior trunk (PO7–13), which is also true for its extinct close relatives (Feldmann et al., 1994; Nagler et al., 2017). Apart from a somewhat elongate body in the type specimens of P. tupiensis and P. circunscriptia, there seems to be not much morphological similarity between U. rostrata and representatives of Protourda. Judging from unpublished images available to us and the type of preservation, it appears doubtful, that there are multiple species of Protourda at the type locality and also a possible synonymy with species of the group Pseudopalaega, recorded from the same locality (Mezzalira and Martins-Neto, 1992; Martins-Neto, 2001), should be considered when revising the material.
Brandt and Poore (2003) interpreted Anthuridea (representatives with long cylindrical bodies) to be the sister group of Gnathiidae, without discussing potential extinct relatives of the group. The morphological features that supported their finding were a reduction of coxal plates (so that they are still present but not visible in dorsal view) and that the vestigial maxilla is conjoined with the paragnaths (‘hypopharynx’ in Brandt and Poore 2003). The former finding appears to be problematic, since the observed condition within Gnathiidae and Anthuridea can be easily explained as a result of a slender body shape and the condition is clearly not true for the larval forms within Gnathiidae (Figure 22F; Wilson et al., 2011, figs. 1A,C, 6A).
Brusca and Wilson (1991) argued for a close relationship between Gnathiidae and Epicaridea because of the similar morphology of the mandible (thin and pointed, molar process absent, palp absent). Dreyer and Wägele (2001), based on molecular data (18S rDNA) found more support for a sister group relationship between Epicaridea and Cymothoidae rather than for a sister group relationship between Gnathiidae and Epicaridea. Nagler et al. (2017) combined the findings of Brusca and Wilson (1991) and Dreyer and Wägele (2001), resulting in a monophyletic group that comprises Cymothoidae, Epicaridea, Gnathiidae. They argued for a closer relationship between Epicaridea and Gnathiidae based on the shared absence of a well-developed maxillula (Brusca and Wilson, 1991). With Urda rostrata and the other extinct species presented herein most likely being the closest known relatives of the group Gnathiidae, the morphology of the extinct relatives of Gnathiidae could provide important morphological data for future phylogenetic analyses.
4.7. PALAEOECOLOGY
Representatives of Urda rostrata and some of its extinct relatives have been discussed to possibly be parasites of fishes (Nagler et al., 2017). Yet, so far there are no publications that could show a direct interaction or association between the crustacean animals and their fish hosts. However, there is one record of representatives of Isopoda that are in direct association with fossil fishes (Nagler et al., 2016). Just as the fossils of Urda rostrata, this record is also from the Tithonian (Upper Jurassic) of the Solnhofen area (southern Germany); yet, the authors of the study did not identify the fossil remains as belonging to U. rostrata. Despite the apomorphic characters of the group that comprises U. rostrata, its extinct relatives and Gnathiidae not being preserved or visible in the figures, some of the fossilized representatives of Isopoda depicted in Nagler et al. (2016) strikingly resemble representatives of Urda rostrata in many aspects.
1) The bodies are of large size compared to other representatives of Isopoda (Nagler et al., 2016, fig. 1). 2) The head appears to be large (in none of the figures it shows much detail; Nagler et al., 2016, fig. 4A). 3) The shape of the legs is similar to that of the herein presented remains of U. rostrata (cf. Nagler et al., 2016, fig. 3A vs. Figure 7A). 4) The shape of the pleotelsa is very similar to that in U. rostrata. Based on the original images (Nagler et al., 2016, figs. 3C, 4A), the pleotelsa appear to be much larger than in the colour-marked reconstructions (Nagler et al., 2016, figs. 3D, 4B) and appear to have a straight mid-part of the posterior margin, like in representatives of U. rostrata (e.g., Figure 1A).
For the fossil remains in Nagler et al. (2016) the tergite of PO7 (‘thoracic segment 2’ therein) is reconstructed to be of the same length as the subsequent tergites (their figs. 2B, 3B, 4B, 5B–C, E). This is in contrast to the herein presented reconstruction of U. rostrata, where this tergite is reconstructed to be short and narrow (Figure 22G). This might be due to a misinterpretation in Nagler et al. (2016), as this structure is not clearly visible in the not-colour-marked figures. Based on the inspection of the figures, one of the fossil remains (Nagler et al., 2016, fig. 4 C–D) might represent a part of the fish rather than a representative of Isopoda, as there appears to be dark, bone-like matter where they interpreted the pleon tergite borders to be located. Nagler et al. (2016, p. 8) interpreted the body of the presumed parasites to be ‘twisted’ as a result of growth response while being permanently attached to their host, similar to extant representatives of Cymothoidae (e.g. Smit et al., 2014). However, none of the ‘twisted’ individuals are accessible in dorsal or ventral view. Therefore, the strongly compressed fossils presented in Nagler et al. (2016) do not allow to unambiguously observe derivations from a strict bilateral symmetry. All associations have in common that the representatives of Isopoda are not randomly distributed on the fish fossils, but all of them are located at the fins and their head is oriented towards the anterior end of the fish (Nagler et al., 2016). With respect to the possibility of rapid oxygen deprivation that has been suggested for at least some of the Solnhofen limestone taphocoenoses (Viohl, 1994; Pan et al., 2019), the occurrences of individuals of U. rostrata on fishes suggests an interaction between living organisms.
As discussed above, the closest relatives of U. rostrata and its herein presented extinct relatives are most likely extant representatives of the group Gnathiidae. Larval forms of all species of Gnathiidae, for which live observations have been made, are parasitic to fishes (Monod, 1926). From this perspective a parasitic lifestyle seems to be a likely feeding mode for their extinct relatives. However, from a pure morphological perspective, the available information is less conclusive.
The eyes in all individuals of U. rostrata, U. mccoyi, U. cretacea, P. suevica and the specimens from the Middle Jurassic of Bielefeld are large and located on the lateral sides of the head, similar to extant larval forms of Gnathiidae, which need to find and attach to their host fishes (Monod, 1926). This suggests that the visual sense likely played an important role in the ecology of the now extinct animals (Nagler et al., 2017). Due to the large size of the fossil specimens, it is likely that they are adult. However, in adult extant parasitic representatives of the group Cymothoidae, which are known to attach to their host for long periods of time, the eyes are often much smaller and proportionally smaller than those of immatures, with adult females having the proportionally smallest eyes, even in species that do not attach within body cavities of the host (e.g. Brusca, 1978; Thamban et al., 2015). In extant representatives of Aegidae, which have been recorded to be temporary parasites of fishes, the eyes of the adults are often very large (Bruce, 2009). This could be an indication that representatives of the above-mentioned extinct species were not permanently attached to the fishes.
Nagler et al. (2017, p. 9) reconstructed the mouthparts of the fossil specimens from Bielefeld (‘Urda rostrata’ therein but see discussion above). They concluded that the mouthparts formed a ‘loose’ mouth-cone and the individual mouthparts were similar to those of extant parasitic forms of the group Cymothoida. Our reconstruction of the mouthparts (based on the same µCT scans) shows important differences to the original reconstruction. 1) Based on our reconstruction, there is no distinct labrum present that could form the anterior confinement of the mouth cone. 2) The maxilliped in our reconstruction corresponds to the maxilla in the reconstruction of Nagler et al. (2017, fig. 4B3). Also, we could not find this structure to have a distal end with 3 spines (Figures 15M, 15N). Overall, in our reconstruction we could not find similar confinement structures as in the feeding apparatus of extant representatives of Cymothoidae or larval forms of Gnathiidae (cf. Figures 15K–N, 10E–I vs. 15A, 15I, 15J). Most importantly, the proportional size and the strongly curved shape of the mandible incisors are very different from the extant parasitic forms within the groups Cymothoidae and Gnathiidae. The shape of the mandibles indicates a piercing rather than a cutting or grinding motion, however, without a sealing mouth-cone, the feeding mechanism of the herein presented fossil specimens remains uncertain.
The morphology of the distal leg elements (dactyli) is different to those in representatives of Cymothoidae, which use their legs to attach to a host. The most obvious difference is that in representatives of Cymothoidae the claws are more strongly curved and the width of the claw at the base is much greater. This would suggest that at least the mechanism of attaching to a fish is different from that in representatives of Cymothoidae and possibly more similar to that in larval forms of Gnathiidae, as they are more similar to these (cf. Figures 14A–F, 16 vs. 20A–B).
4.8. GEOGRAPHIC AND STRATIGRAPHIC DISTRIBUTION
Urda rostrata and all its herein discussed extinct relatives come from Central and Western Europe (Figure 23). Considering the scarcity of the fossil record of the group Isopoda in general, a probably strong geographical sampling bias (intensive collecting in Europe), and the presence of fossils outside of Europe with resemblance to U. rostrata (Taylor, 1972; Grant-Mackie et al., 1996), as of now, the fossil record seems not to be a helpful tool for the study of the biogeographical origin of the group Gnathiidae.
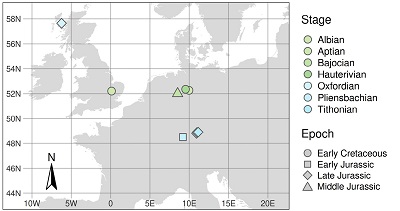 |
| Figure 23. Map of Central Europe with the geographical occurrences of fossil representatives of Urda Münster, 1840 colour and shape coded after the age of the fossils. Map data from naturalearthdata.com (public domain) via ‘rnaturalearth’ (South, 2017). |
The earliest fossils that can be identified as close relatives of Urda rostrata and Gnathiidae are from the Lower Jurassic Amaltheenton Formation (Pliensbachian) in southern Germany (Reiff, 1936; Figure 19). Slightly even older fossils – also from the Pliensbachian – have been found geographically close by (Frentzen, 1937); however, as discussed above, while there are no morphological structures that would argue against a close phylogenetic relationship to U. rostrata, there are not enough structures preserved in the fossils to convincingly argue for a close relationship. The youngest occurrence of a close relative of Urda rostrata, that does not share the above mentioned apomorphies of Gnathiidae, is Albian in age and from the East of England (Carter, 1889; Feldmann et al., 1994) (Figure 19).
When exactly the last relatives of U. rostrata, which are not representatives of Gnathiidae, went extinct is difficult to tell. For once, although there are intensively studied marine sediments from the Upper Cretaceous (e.g. Rathbun, 1935; Lehmann and Höll, 1989), there is no record of animals with a similar body shape, except for one poorly preserved specimen from the Santonian (Upper Cretaceous) of Texas (Bowman, 1971) that bears some resemblance to the herein discussed fossils, but does not allow for a concise systematic interpretation. On the other hand, there is no fossil record of the group Gnathiidae, which could suggest that from the Late Cretaceous on extinct relatives and representatives of Gnathiidae lived in habitats where animals with chitinous exoskeletons are unlikely to be preserved as fossils.
- Taxonomy
Remarks: Full synonymy lists are presented. The style of the synonymy lists and the open nomenclature follows Matthews (1973).
Peracarida Calman, 1904
Isopoda Latreille, 1817
Scutocoxifera Dreyer and Wägele, 2002
Urda Münster, 1840
Type species: Urda rostrata Münster, 1840.
Emended diagnosis: Anterior margin of the head with a straight median portion (proximal joint of the upper lip) and paired concave rounded incisions lateral to it (space for the proximal elements of the antennula); frontal lamina not developed (or conjoined with the head capsule); upper lip large, (can be) projected in anterior direction (not facing in ventro-posterior direction); labrum not distinct (likely conjoined with the clypeus, forming the upper lip); mandible incisor large, projected anteriorly (not to the ventral side), about 90 degrees curved inward, with a pointed tip; tergite of PO7 very short, subsequent tergites much longer; leg of PO7 short and located on the ventral side of the head; pleotelson with lateral sides about parallel in the anterior part, posterior margin semicircular, straight or with a slight concave median notch.
Remarks: The genus Urda was originally described to accommodate four different species (Münster, 1840), all of them recognized later as representing a single species (Oppel, 1862; Kunth, 1870). The genus Reckur was erected by Münster (1842), only to be found synonymous with Urda several decades later (Oppel, 1862; Kunth, 1870). Since then, various isopod fossils from Mesozoic strata were assigned to the genus Urda.
Urda rostrata Münster, 1840
Figures 1–7, 22G
1839 ‘Isopoden’ – Münster, p. 2.
* 1840 Urda rostrata – Münster, p. 21, pl. 1, fig. 2.
1840 Urda decorata – Münster, p. 21, pl. 1, fig. 4.
1840 Urda cincta – Münster, p. 22, pl. 1, fig. 5.
1840 Urda elongata – Münster, p. 22, pl. 1, fig. 3.
. 1842 Reckur punctatus – Münster, p. 77, pl. 9, fig. 10. syn. nov.
1846 ‘Les Urda’ [sic] – Pictet, p. 55, pl. 3, fig. 2.
1846 Reckur affinis – Meyer, p. 598.
1853 Urda decorata Münster – Pictet, atlas, pl. 43, fig. 13.
1854 Urda rostrata Münster – Pictet, p. 467.
1854 Urda decorata Münster – Pictet, p. 467.
1854 Urda cincta Münster – Pictet, p. 467.
1854 Urda elongata Münster – Pictet, p. 467.
1856 Reckur affinis Meyer – Meyer, p. 50, pl. 10, fig. 2.
1862 Urda punctata Münster – Oppel, p .116.
1862 Urda rostrata Münster – Oppel, p. 116.
1870 Urda rostrata Münster – Kunth, p .790, pl. 18, figs. 1, 1a, 2.
. 1870 Urda punctata Münster – Kunth, p. 796, pl. 18, figs. 3, 3a.
1882 Urda rostrata Münster – Ammon, p. 539.
1882 Urda punctata Münster – Ammon, p. 539.
1885 Urda rostrata Münster – Zittel, p. 667, fig. 851.
1887 Urda rostrata Münster – Zittel, p. 664, fig. 868.
1887 Urda punctata Münster – Zittel, p. 664.
1889 Urda rostrata Münster – Carter, p. 194.
1889 Urda punctata Münster – Carter, p. 194.
1904 Urda punctata Münster – Walther, p. 172.
1904 Urda rostrata Münster – Walther, p. 172.
1910 Urda rostrata Münster – Stolley, p. 191.
1910 Urda punctata Münster – Stolley, p. 191.
1912 Urda rostrata Münster – Remeš, p. 176.
1912 Urda punctata (Münster) – Remeš, p. 176.
1928 Urda rostrata Münster – Van Straelen, p. 14.
1928 Urda punctata Münster – Van Straelen, p. 15.
1937 Urda rostrata Münster – Frentzen, p. 102.
1937 Urda punctata Münster – Frentzen, p. 102.
1969 Urda rostrata Münster – Hessler, p. R387.
1971 Urda rostrata Münster – Büchner, p. 32.
1971 Urda punctata Münster – Büchner, p. 32.
1972 Urda rostrata Münster – Taylor, p. 101.
1972 Urda punctata Münster – Taylor, p. 101.
1973 Urda rostrata Münster – Kuhn, fig. 45f.
1988 Urda rostrata Münster – Etter, p. 867.
1988 Urda punctata Münster – Etter, p. 867.
1992 Urda rostrata Münster – Mezzalira and Martins-Neto, p. 55.
1992 Urda punctata Münster – Mezzalira and Martins-Neto, p. 55.
1994 Urda rostrata Münster – Frickhinger, 106, fig. 167.
1996 Urda rostrata Münster – Grant-Mackie, Buckeridge and Johns, p. 37.
1999 Urda rostrata Münster – Brandt, Crame, Polz and Thomson, p. 666, tab. 1.
1999 Urda punctata Münster – Brandt, Crame, Polz and Thomson, p. 666, tab 1.
2014 Urda rostrata Münster – Etter, tab. 1.
2014 Urda punctata Münster – Etter, tab. 1.
2015 Urda rostrata Münster – Schweigert in Arratia, Schultze, Tischlinger and Viohl, p. 289, fig. 602
2017 Urda rostrata Münster – Nagler, Hyžný and Haug, p. 3, tab. 1.
2017 Urda punctata Münster – Nagler, Hyžný and Haug, p. 3, tab. 1.
non 2017 Urda rostrata Nagler, Hyžný and Haug, p. 5, figs. 1A–E, 1G, 2, 3, 4A–C, 5, 6.
Type material studied: Holotype considered lost, not found in the collections in Munich and Berlin, (M. Reich, 2020, pers. comm.; A. Abele-Rassuly, 2021, pers. comm.); holotype of Urda elongata Münster, 1840 (SNSB BSPG AS 493); holotype of Reckur punctatus Münster, 1842 (SNSB BSPG AS 496).
Other material studied: JME SOS 1794; 10 additional specimens from private collections of the German private collector ‘Leptolepides’ (Figure 3), Herbert Gratt (Figure 4A), Manfred Ehrlich (Figure 4B, 4D), Udo Resch (Figures 4C, 5A – E), Falk Starke (Figure 4E), Daniel Fauser (Figure 6), and Norbert Winkler (Figure 7).
Diagnosis: Upper lip distal part wider than proximal part, latero-distal corners rounded; eyes narrow and elongate, tapering towards the posterior end; posterior ends of the eyes close to the level of the posterior margin of the head; tergite of PO7 with convex posterior margin; pleon tergites 1–3 with posterior margin overall concave, convex in the mid-part and concave in the lateral parts; pleotelson posterior margin straight in the median portion.
Remarks: Originally, Meyer (1840) described four different species of Urda, i.e., U. rostrata, U. decorata, U. cincta, and U. elongata. All of them were found synonymous with each other by Oppel (1862). Kunth (1870) recognized Reckur affinis as a junior subjective synonym of U. rostrata. Since then, consistently two species of Urda have been recognized from lithographic limestones of the Solnhofen area, i.e., U. rostrata and U. punctata. Alleged differences are considered as a result of taphonomy (for more details see the text further above). Consequently, both taxa are treated as a single valid species herein. Thus, U. punctata (originally as Reckur punctatus) is herein recognized a junior subjective synonym of U. rostrata.
Occurrence: Upper Jurassic (Tithonian) of Bavaria, Germany.
Urda mccoyi (Carter, 1889)
Figure 8
(1875) Squilla McCoyi – Seeley: museum label. (nomen nudum)
1875 Squilla McCoyi – Jukes-Browne, p. 277.(nomen nudum)
1881 Squilla McCoyi – Jukes-Browne, p. 153. (nomen nudum)
* 1889 Palaega McCoyi – Carter, p. 195, pl. 6, figs. 1–7.
1897 Squilla McCoyi – Cowper Reed, p. 120.
1928 Palaega Mac Coyi Carter – Van Straelen, p. 20.
1994 Urda mccoyi (Carter) – Feldmann, Wieder and Rolfe, p. 88, fig. 2.3, 2.4, 2.6.
non 1994 Urda mccoyi (Carter) – Feldmann, Wieder and Rolfe, p. 88 fig. 2.1, 2.2, 2.5, 2.7.
1999 Urda mccoyi (Carter) – Brandt, Crame, Polz and Thomson, tab. 1.
2006 ?Palaega mccoyi Carter – Feldmann and Rust, tab. 1.
2014 Urda mccoyi (Carter) – Etter, p. 935, tab. 1.
Type material studied: Three syntypes: SM B 23295, SM B 23296, SM B 23297.
Emended diagnosis: Eyes with posterior end at about ⅔ of the heads length; coxal plates of PO8–9 with straight lateral margin parallel to the lateral margin of the tergite; coxal plate of PO10 anterior part wide, posterior part narrower; coxal plates of PO11–13 anterior part narrow, posterior part wider; tergite of PO13 postero-lateral corner pointed or tightly rounded; pleon tergites with lateral parts curved ventrally; pleon tergites 3–4 with posterior margins evenly concave; pleotelson posterior margin rounded (or with a very narrow straight median part, distal-most part not well preserved).
Remarks: The species, originally described as a representative of Palaega, was interpreted to be a representative of Urda by Feldmann et al. (1994), based on the restudy of the type material; we concur with this interpretation. Urda mccoyi differs from the type species, U. rostrata, in having considerably smaller eyes, a proportionally longer tergite of PO8 and rounded posterior margin of the pleotelson. The pleotelson of U. mccoyi is more elongate than in U. buechneri.
Occurrence: Lower Cretaceous (Albian) of England (UK).
Urda aff. mccoyi (Carter, 1889)
1994 Urda mccoyi (Carter) – Feldmann, Wieder and Rolfe, p. 88, fig. 2.1, 2.2, 2.5, 2.7.
Material: One specimen, GSE 15083.
Remarks: There are morphological differences between the specimen from the Isle of Skye and the type material from England. Additionally, the specimen from the Isle of Skye is more than 53 million years older than the type material of U. mccoyi (see discussion above).
Occurrence: Upper Jurassic (lower Oxfordian) of the Isle of Skye (Scotland, UK).
Urda cretacea Stolley, 1910
* 1910 Urda cretacea – Stolley, p. 204, pl. 6. figs. 2–4, 2a–4a.
1914 Urda cretacea Stolley – Calman, p. 325.
1928 Urda cretacea Stolley – Van Straelen, p. 17.
1937 Urda cretacea Stolley – Frentzen, p. 102.
1969 Urda cretacea Stolley – Hessler, p. R387.
1971 Urda cretacea Stolley – Büchner, p. 32.
1972 Urda cretacea Stolley – Taylor, p. 101.
non 1972 Urda cf. cretacea Stolley – Taylor, p. 97, figs. 2.
1988 Urda cretacea Stolley – Etter, p. 865.
1992 Urda cretacea Stolley – Mezzalira and Martins-Neto, p. 55.
1994 Urda cretacea Stolley – Feldmann, Wieder and Rolfe, p. 89.
2017 Urda cretacea Stolley – Nagler, Hyžný and Haug, p. 3, tab. 1.
Type material studied: None. The type material is lost, most likely destroyed in World War II (Nägelke, 2000).
Diagnosis: Eyes with posterior end at about two thirds of the length if the head; upper lip with median process; coxal plates of PO11–12 large, with straight lateral sides parallel to the lateral margins of the tergites, antero-lateral corner angled, postero-lateral corner rounded; pleon tergites with straight posterior margins, lateral parts curved to ventral side; pleon tergites 2–5 with pointed postero-lateral corners.
Remarks: Urda cretacea differs from the type species, U. rostrata, in having shorter eyes, the anterior margin of the upper lip with a median process, the pleotelson with evenly rounded posterior margin. Urda cretacea differs from U. mccoyi in having the head as wide as the tergite of PO8 and the posterior margin of the head in dorsal view being wide.
Taylor (1972) presented a specimen from the Lower Cretaceous of Antarctica, which he identified as Urda cf. cretacea. Feldmann et al. (1994) already noted that they could not support this identification. We concur with Feldmann et al. (1994): the poor preservation of the material from Antarctica precludes an identification of the specimen as a representative of U. cretacea and also as a representative of the group Urda (see discussion above).
Occurrence: Lower Cretaceous (Aptian) of Lower Saxony, Germany.
Urda suevica (Reiff, 1936) n. comb.
Figures 9–11
* 1936 Palaega suevica – Reiff, p. 67, figs. 7a–c, 8, 9; pl. 1, fig. 6–9; pl. 2, fig. 3; fig. 10; pl. 9, figs. 4–6.
. 1936 Palaega kessleri – Reiff, p. 51, fig. 1a–e; pl. 1, figs. 4–5, fig. 2, figs. 3–4; pl. 1, figs. 1–3; pl. 9, figs. 1–9; fig. 5.b syn. nov.
1937 Palaega kessleri Reiff – Frentzen, p. 101.
1968 Palaega kessleri Reiff – Malzahn, p. 832.
1968 Palaega suevica Reiff – Malzahn, p. 832.
1982 Palaega kessleri Reiff – Quayle, p. 31.
1988 Palaega kessleri Reiff – Etter, p. 859.
1988 Palaega suevica Reiff – Etter, p. 859.
1993 Palaega kesslei [sic] Reiff – Obata and Omori, p. 60.
2005 Palaega kessleri Reiff – Feldmann and Goolaerts, p. 1031.
2005 Palaega suevica Reiff – Feldmann and Goolaerts, p. 1031.
2006 Palaega kessleri Reiff – Feldmann and Rust, p. 412, tab. 1.
2006 ?Palaega suevica Reiff – Feldmann and Rust, p. 412, tab. 1.
2013 Palaega kessleri Reiff – Hyžný, Bruce and Schlögl, p. 620.
2013 Palaega suevica Reiff – Hyžný, Bruce and Schlögl, p. 620.
2014 Palaega kessleri Reiff – Etter, p. 935, tab. 1
2013 Palaega suevica Reiff – Etter, p. 935, tab. 1
2014 Palaega kessleri Reiff – Jones, Feldmann and Garassino, p. 740.
2017 Palaega kessleri Reiff – Keupp and Mahlow, p. 162.
2017 Palaega suevica Reiff – Keupp and Mahlow, p. 162.
Neotype: Kirchheimer Exemplar (Fundstück F) in Reiff (1936) collection of the University of Tübingen, GPIT-PV-76948, Lower Jurassic, Pliensbachian, ‘Lias delta’, Amaltheenton Formation, Kirchheim unter Teck, Baden-Württemberg, Germany.
Other material studied: 1 specimen figured in Reiff (1936, ‘Fundstück A’, fig. 1a–c, pl. 1 figs. 4–5) as ‘Palaega kessleri’, GPIT-PV-76947, Reutlingen, Baden-Württemberg, Germany. 1 specimen, figured in Reiff (1936, ‘Fundstück B’, fig. 2) as ‘Palaega kessleri’, collection of the municipal museum of Natural History in Göppingen, without accession number, Holzheim (Göppingen), Baden-Württemberg, Germany. 2 specimens, figured in Reiff (1936; ‘Fundstück C’, figs. 3–4, pl. 1 figs. 1–3, pl. 2 figs. 1–2; ‘Fundstück D’, fig. 5) as ‘Palaega kessleri’, collection of the State Museum of Natural History Karlsruhe, destroyed during World War II (E. Frey, 2020, pers. comm.), Reichenbach (Aalen), Baden-Württemberg, Germany. 1 specimen, figured in Reiff (1936, ‘Fundstück E’, figs. 7–9, pl. 1 figs. 6–9, pl. 2 fig. 3) as ‘Palaega suevica’, collection of the State Museum of Natural History Karlsruhe, destroyed during World War II (E. Frey, 2020, pers. comm.), Holzheim (Göppingen), Baden-Württemberg, Germany. All from the Lower Jurassic, Pliensbachian, ‘Lias delta’, Amaltheenton Formation.
Diagnosis: Eyes with posterior end at about ¾ of the heads length; upper lip with a distinct median convexity; posterior margin of the head convex, without a straight median part; coxal plate of PO8 conjoined with the tergite of PO8.
Remarks: The two names suevica and kessleri were both published in the same publication (Reiff, 1936) with different name bearing types. As discussed above, we find that the two names belong to the same species, making one of the names a subjective synonym of the other. According to ICZN Art. 24.2.2 we give the species suevica precedence over kessleri because GPIT-PV-76948 (part of the type series of suevica) is the only remaining specimen of the two type series where the head is preserve. This makes the name kessleri a subjective synonym of suevica. The holotype of Palaega suevica has been destroyed in WW2 (E. Frey, 2020, pers. comm.). To clarify the taxonomic status of the species, we decided to designate GPIT-PV-76948 (‘Fundstück F’) to be the neotype of the species Urda suevica. Judging from the original description and illustrations, the head morphology in GPIT-PV-76948 is consistent with the head morphology of the (lost) holotype of suevica and the (lost) holotype of kessleri. The holotype of suevica and the neotype of suevica originate from rocks of the same (suggested by the similar preservation) or about the same age (both are specified as ‘Lias delta’) and come from a narrow geographical region (the field sites are less than 20 km apart).
Occurrence: Lower Jurassic (Pliensbachian) of Baden-Württemberg, Germany.
Urda buechneri n. sp.
Figures 12–14, 15K–15N, 16–18
urn:lsid:zoobank.org:act:urn:lsid:zoobank.org:act:DDAF6B55-61EA-491B-959C-569C76B07F1D
1971 Urda sp. Büchner, p. 28, figs. 1–5.
. 2007 ‘Flabellifera’ Wittler, p. 19, fig. 1.
v . 2017 Urda rostrata Nagler, Hyžný and Haug, p. 5, figs. 1A–E, 1G, 2, 3, 4A–C, 5, 6.
Etymology: In honour of Martin Büchner (1932–2022), the former director of the Natural History Museum Bielefeld, who described some of the type specimens in 1971, without formally describing the species.
Holotype: SNSB – BSPG 2011 I 50.
Paratypes: SNSB – BSPG 2011 I 51, ES/jb-8744, ES/jb-30755, ES/jb-30756.
Type location and stratum: Middle Jurassic, Bajocian, Parkinsonia parkinsoni Zone, clay pit ‘Bethel 1’, Bielefeld, North Rhine-Westphalia, Germany.
Diagnosis: Eyes with posterior end at about ¾ of the length of the head; antenna short; tergite of PO7 posterior margin straight; coxal plates of PO8–9 with straight lateral margin parallel to the lateral margins of the tergites; coxal plate of PO10 anterior part wide and much narrower in the posterior part; coxal plates of PO11–13 anterior part narrow and posterior part wider; tergite of PO13 with postero-lateral corner widely rounded; pleon tergites with about straight posterior margins; pleon tergites 2–5 with lateral parts curved to the ventral side, postero-lateral corners pointed and projecting posteriorly; pleotelson posterior margin rounded; uropod endopod lateral margin with denticles.
Remarks: The type material of U. buechneri n. sp. has previously been figured as U. rostrata (Nagler et al., 2017); the same material is herein interpreted as a belonging to a species distinct from U. rostrata. Urda buechneri n. sp. differs from U. rostrata in having distinctly shorter eyes (relative to the length of the head) and from U. mccoyi in having a less bulged head and a less convex posterior margin of the tergite of PO7. Urda buechneri n. sp. differs from U. cretacea in having a narrower head and from U. suevica n. comb. in having a straight mid-part in the posterior margin of the head and in having distinct coxal plates in PO8.
Occurrence: Middle Jurassic (Bajocian) of North Rhine-Westphalia, Germany.
Urda stemmerbergensis (Malzahn, 1968) n. comb.
* 1968 Palaega? stemmerbergensis Malzahn, p. 828, pl. 58, figs. 1–2, 4–5.
1975 Palaega stemmerbergensis Malzahn – Secretan, p. 320.
2005 ?Palaega stemmerbergensis Malzahn – Feldmann and Goolaerts, p. 1031.
2006 ?Palaega stemmerbergensis Malzahn – Feldmann and Rust, p. 412, tab. 1.
2015 Palaega stemmerbergensis Malzahn – Vonk, Latella and Zorzin, p. 543.
Type material studied: None. The type material consisting of a single specimen has to be considered lost (C. Heunisch, 2019, pers. comm.).
Remarks: The affinity of this fossil with other representatives of Palaega (collective group) has already been doubted in its original description (Malzahn, 1968) – e.g., the pleotelson in U. stemmerbergensis lacks a spinose posterior margin which has been one of the most important characters for the assignment of species to Palaega (Hyžný et al., 2013). The holotype of U. stemmerbergensis shares multiple characters with species of Urda as characterised herein (for more details see discussion above). The poor preservation of the single type specimen does not allow to differentiate the species from other species of Urda as herein characterised. The detailed nature of the description and the photographs provided in the original publication should, however, allow to relate potential future specimens to the holotype.
Occurrence: Lower Cretaceous (Hauterivian) of Lower Saxony, Germany.
Scutocoxifera incertae sedis
Eobooralana n. gen.
urn:lsid:zoobank.org:act:2478BBC2-266E-4A4B-949B-251819A41FDA
Etymology: Prefix eo (from Greek ēōs, meaning dawn) refers to the age of the holotype of the type species; -booralana indicates the superficial resemblance to the extant species Booralana tricarinata Camp and Heard, 1988, which etymological origin is the aboriginal word booral, meaning large reflecting the size of the holotype of the type species; the gender is feminine.
Type species: Eobooralana rhodanica (Van Straelen, 1928) n. comb.
Diagnosis: As for the species/not applicable, since monotypic.
Remark: The holotype of the type species cannot be identified to a group ranked at genus level based on apomorphic character states. To be consistent with the recommendations of the ICZN, this new generic name is provided.
Eobooralana rhodanica (Van Straelen, 1928) n. comb.
* 1928 Urda rhodanica – Van Straelen, p. 13, text fig. 1, pl. 1, fig. 1.
1988 Urda rhodanica Van Straelen – Etter, p. 867.
1992 Urda rhodanica Van Straelen – Mezzalira and Martins-Neto, p. 55.
1999 Urda rhodanica Van Straelen – Brandt, Crame, Polz and Thomson, tab. 1.
2014 Urda rhodanica Van Straelen – Etter, tab. 1.
2017 Urda rhodanica Van Straelen – Nagler, Hyžný and Haug, p. 3, tab. 1.
Type material studied: Interpretation based on Van Straelenʼs text fig. 1 (drawing) and pl. 1, fig. 1 (photograph); type material should be located in the collection of the Institut de Géologie de I’Université de Lyon.
Diagnosis: Coxal plates of PO10–13 (all that are preserved) with transverse furrow in the anterior part; coxal plates of PO10–11 of about the same size; coxal plates of PO11–13 increasing in size; pleotelson about as long as coxal plate of PO13, in the anterior part with an elevation orthogonal to the midline, with a carina along the midline posterior to the elevation, posterior margin concave in the median part; uropod endopod and exopod distally extending to the level of the pleotelson posterior margin.
Remarks: Although the only known specimen of Eobooralana rhodanica n. comb. does not possess the head and the anterior portion of the trunk, the coxal plate of PO12 is much larger than the coxal plate of PO11 and the coxal plate PO13 is even larger than the coxal plate of PO12, whereas in the type species of Urda (U. rostrata) and its congeners (U. buechneri n. sp., U. cretacea, U. mccoyi) the coxal plate of PO13 is smaller than the preceding coxal plates. Additionally, the posterior margin of the pleotelson in E. rhodanica n. comb. has a distinct concave notch, which is much more prominent than that in the type species of Urda, U. rostrata.
Occurrence: Middle Jurassic (Callovian) of France.
Scutocoxifera incertae sedis
Urda? liasica (Frentzen, 1937) nom. dub.
* 1937 Urda liasica – Frentzen, p. 101, text fig. 1b.
1972 Urda liasica Frentzen – Taylor, p. 101.
1988 Urda liasica Frentzen – Etter, p. 867.
1992 Urda liasica Frentzen – Mezzalira and Martins-Neto, p. 55.
1999 Urda liasica Frentzen – Brandt, Crame, Polz and Thomson, tab. 1.
2014 Urda liasica Frentzen – Etter, tab. 1.
2017 Urda liasica Frentzen – Nagler, Hyžný and Haug, p. 3, tab. 1.
Type material studied: None. The type material, consisting of a single specimen, is reported to have been destroyed during World War II (E. Frey, 2020, pers. comm.).
Remarks: Neither the description nor the accompanying single, rather stylised, drawing of the holotype allow to relate any new material that might emerge in the future to the holotype. Only the implausible recovery of the holotype could make it possible to to apply the name Urda? liasica to an actual population in an undoubtful manner. Consequently, Urda? liasica is herein considered a nomen dubium.
Occurrence: Lower Jurassic (Toarcian) of Baden-Württemberg, Germany.
Urda? moravica (Remeš, 1912)
* 1912 Urda moravica Remeš, p. 173, pl. 1, figs. 1–4.
1928 Urda moravica Remeš – Van Straelen, p. 14.
1972 Urda moravica Remeš – Taylor, p. 101.
1988 Urda moravica Remeš – Etter, p. 867.
1992 Urda moravica Remeš – Mezzalira and Martins-Neto, p. 55.
1999 Urda moravica Remeš – Brandt, Crame, Polz and Thomson, tab. 1.
2014 Urda moravica Remeš – Etter, tab. 1.
2017 Urda moravica Remeš – Nagler, Hyžný and Haug: p. 3, tab. 1.
Type material studied: None. The type material was supposed to be deposited in the palaeontological collections of the University of Vienna. The search at the respective institution by one of us (MH) was not successful; hence, the type material of Urda? moravica is considered lost.
Remarks: Although the type material is lost, it should be possible to relate future specimens that might emerge from the same locality or nearby to the holotype, since its description is accompanied by photographs and a seemingly accurate drawing. With the preservation of the single known specimen, consisting only of the posterior part of the body, it is currently impossible to reliably differentiate Urda? moravica from other species within Scutocoxifera.
Occurrence: Middle Jurassic (Bathonian) of the Chřiby mountain region, Czech Republic.
Urda? zelandica (Buckeridge and Johns in Grant-Mackie, Buckeridge and Johns, 1996)
* 1996 Urda zelandica Buckeridge and Johns in Grant-Mackie, Buckeridge and Johns, p. 35, figs. 3–5.
1999 Urda zelandica Buckeridge and Johns – Brandt, Crame, Polz and Thomson, tab. 1.
2014 Urda zelandica Buckeridge and Johns – Etter, tab. 1.
2017 Urda zelandica Buckeridge and Johns – Nagler, Hyžný and Haug, p. 3, tab. 1.
Type material studied: Holotype: A406 in collection of the Geology Department, University of Auckland.
Remarks: Despite the resemblance to the fossils of U. rostrata and the species herein interpreted as close relatives, the holotype, which is a strongly compressed fossil of only the posterior body region, does not yield enough characters to confirm a close relationship with U. rostrata. It is currently not possible to reliably differentiate Urda? zelandica from other species.
Occurrence: Upper Jurassic (Tithonian) of North Island, New Zealand.
- Conclusions
- There is only a single species – Urda rostrata– that occurs in the Late Jurassic limestones of the Solnhofen area (southern Germany).
- The fossil specimens from the Middle Jurassic of Bielefeld are not conspecific with rostratabut can be attributed to a new species: Urda buechneri n. sp.
- Several species that have been attributed to the genus Urdacannot be safely identified as close relatives of the type species rostrata or cannot be distinguished from other species.
- Urda rostrataand its extinct relatives are closely related to the group Gnathiidae.
- There is no autapomorphy for a monophyletic group Urda, but there are apomorphic character states for an unnamed group that comprises Gnathiidae and all species herein attributed to the name Urda.
- Well preserved fossils, as the ones presented herein, could play an important role to determine the phylogenetic position of the group Gnathiidae within its parent group Scutocoxifera.
- All fossil remains that can clearly be identified as belonging to close relatives of rostrataare from Europe with a stratigraphic range spanning from the Early Jurassic to the Early Cretaceous (ca. 185–105 million years before present).
Author contributions
MS designed the study, contributed photographs and µCT data, performed 3D reconstructions, designed the figures and contributed most parts of the main text. CN provided photographs and µCT data. MH contributed to the study design and contributed to all parts of the manuscript. All authors reviewed the final manuscript.
Financing
This study is part of the project Palaeo-Evo-Devo of Malacostraca kindly funded by the Deutsche Forschungsgesellschaft (DFG 6300/3-2). CN was funded by the Studienstiftung des Deutschen Volkes with a PhD fellowship. MH was supported by VEGA/02/0169/19 and the Slovak Research and Development Agency under the contract no. APVV-17-0555.
Acknowledgements
Foremost we would like to thank Joachim T. Haug (LMU Munich) who supervised the doctoral dissertation of MS, of which this contribution is a part of, and who provided guidance and valuable comments that drastically improved the manuscript. We are grateful to Günter Schweigert (State Museum of Natural History Stuttgart) and Rodney M. Feldmann (Kent State University) for their constructive criticism as reviewers. We thank the curators Mark Keiter (Natural History Museum Bielefeld), Mike Reich (Staatliches Naturhistorisches Museum Braunschweig), Alexander Nützel and Martin Nose (both BSPG, Bavarian State Collection of Palaeontology, Munich), Ingmar Werneburg (University of Tübingen), Günter Schweigert (SMNS, State Museum of Natural History Stuttgart), Enrico Schwabe, and Stefan Friedrich (ZSM, Zoological State Collection Munich) and Martin Schwentner (Natural History Museum Vienna, formerly CeNak Hamburg) for loaning fossil and extant specimens. We thank Andreas Abele-Rassuly (Museum für Naturkunde Berlin) for providing high-quality photographs of fossils and metadata. We thank Carmen Heunisch (Landesamt für Bergbau, Energie und Geologie Niedersachsen) for searching for a lost type specimen. We thank Roland Melzer (ZSM, Zoological State Collection Munich) for providing access to the µCT facility and help with the scanning. We thank Nadine Usimesa Wingi (ZSM) for her help with the fluorescence imaging. The Steinkern forum (https://forum.steinkern.de) allowed to conveniently contact numerous fossil collectors, who made this study possible by providing photographs, loaning or providing access to their fossils (in alphabetical order): Achim Hildebrand (Spenge), Alfred Walkowiak (Hausen, Forchheim, Germany), Daniel Fauser (Schwäbisch Gmünd, Germany), Falk Starke (Bodenwerder), Herbert Gratt (Brixlegg), Jürgen Graf (Künzelsau), Manfred Ehrlich (Böhl-Iggelheim), Norbert Winkler (Stahnsdorf), ‘Leptolepides’, Udo Resch (Eichstätt), Volker Kurth (Ludwigsburg). Paula Pazinato (LMU Munich), Rosemarie Rohn Davies and William Sallun Filho (both São Paulo State University), for providing photographs of the Protourda material. We thank Carolin Haug and J. M. Starck (both Munich) for longstanding support. Margarita Yavorskaya (University of Tübingen) helped with translations and provided valuable suggestions. Thanks to numerous contributors, most of the digital work could be done using free and open-source software.
References
Abd El-Atti, M., 2020, Light and Scanning electron microscopic investigations on gravid female Anilocra sp. (Isopoda: Cymothoidae) infesting Tilapia zillii in Qarun Lake, Egypt: Egyptian Journal of Aquatic Biology and Fisheries 24(5), 183–196. https://doi.org/10.21608/ejabf.2020.104225
Ammon, L. von, 1882, Ein Beitrag zur Kenntnis der fossilen Asseln: Sitzungsberichte der Mathematisch-Physikalischen Classe der Königlichen Bayerischen Akademie der Wissenschaften, 12, 507–551.
An, J., Boyko, C.B., Li, X., 2015, A review of bopyrids (Crustacea: Isopoda: Bopyridae) parasitic on caridean shrimps (Crustacea: Decapoda: Caridea) from China: Bulletin of the American Museum of Natural History, 399, 01–85. https://doi.org/10.1206/amnb-921-00-01.1
Ax, P., 2000, Multicellular animals. The phylogenetic system of the metazoa: Berlin, Germany, Springer-Verlag, 154 p. https://doi.org/10.1007/978-3-662-10396-8
Barnard, K.H., 1920, Contributions to the crustacean fauna of South Africa. No. 6.- Further additions to the list of marine Isopoda: Annals of the South African Museum, 17(5), 319–438. https://doi.org/10.5962/bhl.part.22318
Bengtson, S., 2000, Teasing fossils out of shales with cameras and computers: Palaeontologia Electronica 3(1), 1–14.
Bowman, T.E., 1971, Palaega lamnae, new species (Crustacea: Isopoda) from the Upper Cretaceous of Texas: Journal of Paleontology 45(3), 540–541.
Boyko, C.B., Wolff, C., 2014, Isopoda and Tanaidacea: in Martin, J.W., Olesen, J., Hoeg, J.T. (eds.), Atlas of Crustacean Larvae: Baltimore, Johns Hopkins University Press, 210–212.
Brandt, A., Poore, G.C., 2001, Two new species of Tridentella (Crustacea: Isopoda: Tridentellidae) from Namibia: Beaufortia 51(11), 199–212.
Brandt, A., Poore, G.C., 2003, Higher classification of the flabelliferan and related Isopoda based on a reappraisal of relationships: Invertebrate Systematics 17(6), 893–923. https://doi.org/10.1071/IS02032
Bruce, N.L., 1986, Cirolanidae (Crustacea: Isopoda) of Australia: Records of the Australian Museum, Supplement 6, 239p. https://doi.org/10.3853/j.0812-7387.6.1986.98
Bruce, N.L., 2005, Two new species of the mesopelagic isopod genus Syscenus Harger, 1880 (Crustacea: Isopoda: Aegidae) from the southwestern Pacific: Zootaxa 1070(1), 31–42. https://doi.org/10.11646/zootaxa.1070.1.2
Bruce, N.L., 2009, The marine fauna of New Zealand: Isopoda, Aegidae (Crustacea), Biodiversity Memoir: Wellington, National Institute of Water and Atmospheric Research.
Bruce, N.L., Olesen, J., 2002, Cirolanid isopods from the Andaman Sea off Phuket, Thailand, with description of two new species: Phuket Marine Biological Center Special Publication 23(1), 109–131.
Brusca, R.C., 1978, Studies on the cymothoid fish symbionts of the eastern Pacific (Isopoda, Cymothoidae) I. Biology of Nerocila californica: Crustaceana 34(2), 141–154. https://doi.org/10.1163/156854078X00718
Brusca, R.C., Wilson, G.D.F., 1991, A phylogenetic analysis of the Isopoda with some classificatory recommendations: Memoirs of the Queensland Museum 31, 143–204.
Büchner, M., 1971, Eine fossile Meeresassel (Isopoda, Malacostraca) aus den Parkinsonienschichten (Mittlerer Jura) von Bethel, Kreis Bielefeld: Berichte des Naturwissenschaftlichen Vereins Bielefeld 27–35.
Calman, W.T., 1914, Reviews. I.-Fossil Crustacea: Geological Magazine 1(7), 325–326. https://doi.org/10.1017/S0016756800139767
Camp, D.K., 1988, Bythognathia yucatanensis, new genus, new species, from Abyssal depths in the Caribbean Sea, with a list of gnathiid species described since 1926 (Isopoda: Gnathiidea): Journal of Crustacean Biology 8(4), 668–678. https://doi.org/10.1163/193724088X00503
Camp, D.K., Heard, R.W., 1988, Booralana tricarinata, A new species of isopod from the Western Atlantic Ocean (Crustacea, Isopoda, Cirolanidae): Proceedings of the Biological Society of Washington 101(3), 603–613.
Cardona, A., Saalfeld, S., Schindelin, J., Arganda-Carreras, I., Preibisch, S., Longair, M., Tomancak, P., Hartenstein, V., Douglas, R.J., 2012, TrakEM2 Software for Neural Circuit Reconstruction: PLOS ONE 7(6), e38011. https://doi.org/10.1371/journal.pone.0038011
Carter, J., 1889, On fossil isopods, with a description of a new species: The Geological Magazine, New Series 3(6), 193–196. https://doi.org/10.1017/S0016756800189083
Cohen, B.F., Poore, G.C.B., 1994, Phylogeny and biogeography of the Gnathiidae (Crustacea: Isopoda) with descriptions of new genera and species, most from southeastern Australia: Memoirs of the Museum of Victoria 54(2), 271–397. https://doi.org/10.24199/j.mmv.1994.54.13
Cowper Reed, F.R., 1897, The Geology of Cambridgeshire: Cambridge, UK, Cambridge University Press, 276 p.
Daniel, G., Nilsson, T., Cragg, S., 1991, Limnoria lignorum ingest bacterial and fungal degraded wood: Holz als Roh- und Werkstoff 49(12), 488–490. https://doi.org/10.1007/BF02619480
Delaney, P.M., 1989, Phylogeny and biogeography of the marine isopod family Corallanidae (Crustacea, Isopoda, Flabellifera): Contributions in Science 409, 75p.
Dreyer, H., Wägele, J.-W., 2001, Parasites of crustaceans (Isopoda: Bopyridae) evolved from fish parasites: molecular and morphological evidence: Zoology 103(3-4), 157–178.
Dreyer, H., Wägele, J.-W., 2002, The Scutocoxifera tax. nov. and the information content of nuclear ssu rDNA sequences for reconstruction of isopod phylogeny (Peracarida: Isopoda): Journal of Crustacean Biology 22(2), 217–234. https://doi.org/10.1163/20021975-99990229
Eklund, M.J., Aase, A., Bell, C.J., 2018, Progressive photonics: methods and applications of sequential imaging using visible and non-visible spectra to enhance data-yield and facilitate forensic interpretation of fossils: Journal of Paleontological Techniques 20, 1–36.
Etter, W., 1988, Isopoden und Tanaidaceen (Crustacea, Malacostraca) aus dem unteren Opalinuston der Nordschweiz: Eclogae geologicae Helvetiae 81, 857–877. https://doi.org/10.5169/seals-166204
Etter, W., 2014, A well-preserved isopod from the Middle Jurassic of southern Germany and implications for the isopod fossil record: Palaeontology, 57(5), 931-949. https://doi.org/10.1111/pala.12095
Fedorov, A., Beichel, R., Kalpathy-Cramer, J., Finet, J., Fillion-Robin, J.-C., Pujol, S., Bauer, C., Jennings, D., Fennessy, F., Sonka, M., Buatti, J., Aylward, S., Miller, J.V., Pieper, S., Kikinis, R., 2012, 3D Slicer as an image computing platform for the Quantitative Imaging Network: Magnetic Resonance Imaging, Quantitative Imaging in Cancer 30(9), 1323–1341. https://doi.org/10.1016/j.mri.2012.05.001
Feldmann, R.M., Schweitzer, C.E., Maxwell, P.A., Kelley, B.M., 2008, Fossil isopod and decapod crustaceans from the Kowai Formation (Pliocene) near Makikihi, South Canterbury, New Zealand: New Zealand Journal of Geology and Geophysics 51(1), 43–58. https://doi.org/10.1080/00288300809509849
Feldmann, R.M., Wieder, R.W., Ian Rolfe, W.D., 1994, Urda mccoyi (Carter 1889), an isopod crustacean from the Jurassic of Skye: Scottish Journal of Geology 30(1), 87–89. https://doi.org/10.1144/sjg30010087
Fraser, D., Mallon, J.C., Furr, R., Theodor, J.M., 2009, Improving the repeatability of low magnification microwear methods using high dynamic range imaging: PALAIOS 24(12), 818–825. https://doi.org/10.2110/palo.2009.p09-064r
Frentzen, K., 1937, Paläontologische Notizen aus den Badischen Landessamlungen für Naturkunde, Karlsruhe i. B. I. Neue Funde von Isopoden (Asseln) im Lias Südwestdeutschlands: Beiträge zur Naturkundlichen Forschung in Südwestdeutschland 2, 100–103.
Frickhinger, K.A., 1994, Die Fossilien von Solnhofen: Germany, Goldschneck-Verlag, 336 p.
Gantt, H.L., 1910, Work, Wages and Profit, 2a ed: New York, The Engineering Magazine Co., 312 p.
Gearty, W., 2021, deeptime: Plotting tools for anyone working in deep time: R.
Grant-Mackie, J.A., Buckeridge, J.S., Johns, P.M., 1996, Two new Upper Jurassic arthropods from New Zealand: Alcheringa: An Australasian Journal of Palaeontology 20(1), 31–39. https://doi.org/10.1080/03115519608619221
Grobe, P., Vogt, P.R., 2009, Morph.D.Base 2.0: A public data base for morphological data, metadata, and phylogenetic matrices. available at: http://www.morphdbase.de
Haack, W., 1933, Zur Verbreitung des Asselkrebses Archaeoniscus brodiei M. EDW. im Serpulit des Teutoburger Waldes: Zeitschrift der Deutschen Geologischen Gesellschaft 85, 229–234.
Haug, C., Haug, J.T., Waloszek, D., Maas, A., Frattigiani, R., Liebau, S., 2009, New methods to document fossils from lithographic limestones of Southern Germany and Lebanon: Palaeontologia Electronica 12(3), 1–12.
Haug, C., Kutschera, V., Ahyong, S., Vega, F., Maas, A., Waloszek, D., Haug, J., 2013, Re-evaluation of the Mesozoic mantis shrimp Ursquilla yehoachi based on new material and the virtual peel technique: Palaeontologia Electronica 16(2), 1–14. https://doi.org/10.26879/340
Haug, J.T., Haug, C., 2011, Fossilien unter langwelligem Licht: Grün-Orange-Fluoreszenz an makroskopischen Objekten: Archaeopteryx 29, 20–23.
Haug, J.T., Haug, C., Kutschera, V., Mayer, G., Maas, A., Liebau, S., Castellani, C., Wolfram, U., Clarkson, E.N.K., Waloszek, D., 2011, Autofluorescence imaging, an excellent tool for comparative morphology: Journal of Microscopy 244(3), 259–272. https://doi.org/10.1111/j.1365-2818.2011.03534.x
Haug, J.T., Maas, A., Waloszek, D., 2010, †Henningsmoenicaris scutula, †Sandtorpia vestrogothiensis gen. et sp. nov. and heterochronic events in early crustacean evolution: Transactions of the Royal Society of Edinburgh 100(3), 311–350. https://doi.org/10.1017/S1755691010008145
Hesse, E., 1864, Les Pranizes et sur les Ancées: Mémoire sur les pranizes et les ancées et sur les moyens curieux à l’aide desquels certains crustacés parasites assurent la conservation de leur espèce, Des Mémoires Presentés Par Divers Savants à L’Institut Impérial De France, Paris, Impremerie Imperiale, 72p.
Hessler, R.R., 1969, Peracarida, in Moore, R.C. (ed.), Treatise of Invertebrate Paleontology, Part R, Arthropoda 4: Kansas, The Geological Society of America and University of Kansas, R360–R393.
Hopson, P.M., Wilkinson, I.P., Woods, M.A., 2008, A stratigraphical framework for the Lower Cretaceous of England: British Geological Survey, Research Report(RR/08/03), 87p.
Hyžný, M., Bruce, N.L., Schlögl, J., 2013, An appraisal of the fossil record for the Cirolanidae (Malacostraca: Peracarida: Isopoda: Cymothoida), with a description of a new cirolanid isopod crustacean from the early Miocene of the Vienna Basin (Western Carpathians): Palaeontology 56(3), 615–630. https://doi.org/10.1111/pala.12006
Itoh, K., Hayashi, A., Ichioka, Y., 1989, Digitized optical microscopy with extended depth of field: Applied Optics 28(16), 3487–3493. https://doi.org/10.1364/AO.28.003487
Jukes-Browne, A.J., 1875, On the relations of the Cambridge Gault and Greensand: Quarterly Journal of the Geological Society 31, 256–316. https://doi.org/10.1144/GSL.JGS.1875.031.01-04.19
Jukes-Browne, A.J., 1881, Appendix B. List of Gault Fossils (remaniés) found in the “Cambridge Greensand”, in Penning, W.H., Jukes-Browne, A.J. (eds.), The Geology of the Neighbourhood of Cambridge: London, H.M. Stationery Office, 149–154.
Kerp, H., Bomfleur, B., 2011, Photography of plant fossils—New techniques, old tricks: Review of Palaeobotany and Palynology 166(3–4), 117–151. https://doi.org/10.1016/j.revpalbo.2011.05.001
Keupp, H., Mahlow, K., 2017, Eine neue Isopoden-Art (Palaega johannschoberti n. sp.) aus dem Amaltheenton (Unter-Jura, Ober-Pliensbachium) von Buttenheim in Oberfranken: Zitteliana 89, 161–170. https://doi.org/10.5282/ubm/epub.40462
Kikinis, R., Pieper, S.D., Vosburgh, K.G., 2014, 3D Slicer: A Platform for Subject-Specific Image Analysis, Visualization, and Clinical Support, in Jolesz, F.A. (ed.), Intraoperative Imaging and Image-Guided Therapy: New York, Springer, 277–289. https://doi.org/10.1007/978-1-4614-7657-3_19
Kuhn, O., 1973, Die Tierwelt des Solnhofener Schiefers, Neue Brehm-Bücherei, vol. 318: Lutherstadt Wittenberg, Germany, A. Ziemsen, 119 p.
Kunth, A., 1870, Ueber wenig bekannte Crustaceen von Solenhofen: Zeitschrift der Deutschen Geologischen Gesellschaft 22(4), 771–802.
Kussakin, O.G., Rybakov, A.V., 1995, Protognathia waegeli sp. n. (Crustacea, Isopoda, Flabellifera), a new species from the Antarctic of the rare and poorly known family Protognathiidae: Russian Journal of Marine Biology 21(1), 17–28.
Lanham, U., 1965, Uninominal nomenclature: Systematic Zoology, 14(2), 144–144. https://doi.org/10.2307/2411739
Lehmann, J., Höll, K., 1989, Asseln aus dem Cenoman (Oberkreide) Nordwestdeutschlands: Arbeitskreis Paläontologie Hannover 17(1), 1–16.
Limaye, A., 2012, Drishti: a volume exploration and presentation tool, in Stock, S.R. (ed.), presented at SPIE Optical Engineering + Applications: California, USA, SPIE Digital Library, 1–10. https://doi.org/10.1117/12.935640
Lins, L.S.F., Ho, S.Y.W., Wilson, G.D.F., Lo, N., 2012, Evidence for Permo-Triassic colonization of the deep sea by isopods: Biology Letters 8(6), 979–982. https://doi.org/10.1098/rsbl.2012.0774
Lösel, P.D., van de Kamp, T., Jayme, A., Ershov, A., Faragó, T., Pichler, O., Tan Jerome, N., Aadepu, N., Bremer, S., Chilingaryan, S.A., Heethoff, M., Kopmann, A., Odar, J., Schmelzle, S., Zuber, M., Wittbrodt, J., Baumbach, T., Heuveline, V., 2020, Introducing Biomedisa as an open-source online platform for biomedical image segmentation: Nature Communications 11(1), 5577. https://doi.org/10.1038/s41467-020-19303-w
Luque, J., Schweitzer, C.E., Santana, W., Portell, R.W., Vega, F.J., Klompmaker, A.A., 2017, Checklist of fossil decapod crustaceans from tropical America. Part I: Anomura and Brachyura: Nauplius 25,e2017025. https://doi.org/10.1590/2358-2936e2017025
Malzahn, E., 1968, Über einen neuen Isopoden aus dem Hauterive Nordwestdeutschlands: Geologisches Jahrbuch 86, 827–834.
Manship, B.M., Walker, A.J., Davies, A.J., 2011, Brooding and embryonic development in the crustacean Paragnathia formica (Hesse, 1864) (Peracarida: Isopoda: Gnathiidae): Arthropod Structure & Development 40(2), 135–145. https://doi.org/10.1016/j.asd.2010.12.004
Martins-Neto, R.G., 2001, Review of some Crustacea (Isopoda and Decapoda) from Brazilian deposits (Paleozoic. Mesozoic and Cenozoic) with descriptions of new taxa: Acta Geologica Leopoldensia 24(52/53), 237–254.
Matthews, 1973, Notes on open nomenclature and synonymy lists: Palaeontology 16(4), 713–719.
Menzies, R.J., 1962, The isopods of abyssal depths in the Atlantic Ocean: Vema Research Series 1, 79–206.
Messana, G., 2020, Catailana whitteni, a new genus and species of stygobiotic cirolanid from a cave in Guangxi, China (Crustacea: Isopoda: Cirolanidae): Raffles Bulletin of Zoology, 35, 101–108.
Meyer, H. von, 1846, Mittheilungen an Professor Bronn gerichtet: Neues Jahrbuch für Mineralogie, Geognosie, Geologie und Petrefaktenkunde 1846, 596–599.
Meyer, H. von, 1856, Jurasische und Triasische Crustaceen: Palaeontographica 4(2), 44–55.
Meyer, H. von., 1840, Beiträge zur Petrefacten Kunde: Buchner’sche Buchhandlung, Bayreuth, 152 p.
Mezzalira, S., Martins-Neto, R.G., 1992, Novos crustáceos Paleozóicus do estado de Sao Paulo, com descricao de novos taxa: Acta Geologica Leopoldensia 36(15), 49–66.
Mones, A., 1989, Nomen dubium vs. nomen vanum: Journal of Vertebrate Paleontology 9(2), 232–234.
Monod, T., 1926, Les Gnathiidæ. Essai monographique (Morphologie, Biologie, Systématique): Mémoires de la Société des Sciences Naturelles du Maroc 13, 668p.
Münster, G.G. zu, 1839, Ueber die fossilen langschwänzigen Krebse in den Kalkschiefern von Bayern: Beiträge zur Petrefacten-Kunde 2, 88p.
Münster, G.G. zu, 1840, Ueber einige Isopoden in den Kalkschiefern von Bayern: Beiträge zur Petrefacten-Kunde 3, 19–23.
Münster, G.G., 1842, Beschreibung drei neuer Arten Crustaciten: Beiträge zur Petrefacten-Kunde 5, 76–78.
Nägelke, H.D., 2000, Hochschulbau im Kaiserreich: historische Architektur im Prozess bürgerlicher Konsensbildung: Kiel, Verlag Ludwig, 520 p.
Nagler, C., Haug, C., Resch, U., Kriwet, J., Haug, J.T., 2016, 150 million years old isopods on fishes: a possible case of palaeo-parasitism: Bulletin of Geosciences 91(1), 1–12. https://doi.org/10.3140/bull.geosci.1586
Nagler, C., Haug, J.T., 2016, Functional morphology of parasitic isopods: understanding morphological adaptations of attachment and feeding structures in Nerocila as a pre-requisite for reconstructing the evolution of Cymothoidae: PeerJ, 4, e2188. https://doi.org/10.7717/peerj.2188
Nagler, C., Hyžný, M., Haug, J.T., 2017, 168 million years old “marine lice” and the evolution of parasitism within isopods: BMC Evolutionary Biology 17(1), 76. https://doi.org/10.1186/s12862-017-0915-1
Nozères, C., 2008, Aega psora (Linnaeus, 1758), in Boyko, C.B., Bruce, N.L., Hadfield, K.A., Merrin, K.L., Ota, Y., Poore, G.C.B., Taiti, S., Schotte, M., Wilson, G.D.F., (eds.), World Marine, Freshwater and Terrestrial Isopod Crustaceans database, World Register of Marine Species. https://doi.org/10.14284/365
Ogg, J.G., Ogg, G., Gradstein, F.M., 2016, A concise geologic time scale 2016: Amsterdam, Elsevier, 234 p.
Oppel, A., 1862, Ueber jurassische Crustaceen Palaeontologische Mittheilungen aus dem Museum des Koeniglich Bayerischen Staates 1, Germany, Nabu Press, 120p. https://doi.org/10.5962/bhl.title.15040
Ota, Y., 2014, Three new gnathiid species with larvae ectoparasitic on coastal sharks from southwestern Japan (Crustacea: Isopoda): Zootaxa 3857(4), 478-500. https://doi.org/10.11646/zootaxa.3857.4.2
Ota, Y., 2019, Description of female adult and praniza larva of Tenerognathia visus Tanaka, 2005 (Crustacea; Isopoda; Gnathiidae) with notes on mating behavior: Zootaxa 4711(3), 561–570. https://doi.org/10.11646/zootaxa.4711.3.7
Ota, Y., Hirose, E., 2009, Gnathia nubila n. sp. and a new record of Gnathia grandilaris (Crustacea, Isopoda, Gnathiidae) that parasitizes elasmobranchs from Okinawan coastal waters, Japan: Zootaxa 2238(1), 43–55. https://doi.org/10.11646/zootaxa.2238.1.4
Owen, H.G., 2002, The base of the Albian Stage; comments on recent proposals: Cretaceous Research 23(1), 1–13. https://doi.org/10.1006/cres.2001.0306
Pan, Y., Fürsich, F.T., Chellouche, P., Hu, L., 2019, Taphonomy of fish concentrations from the Upper Jurassic Solnhofen Plattenkalk of Southern Germany: Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen 292(1), 73–92. https://doi.org/10.1127/njgpa/2019/0809
Pebesma, E., 2018, Simple Features for R: Standardized Support for Spatial Vector Data: The R Journal 10(1), 439-446. https://doi.org/10.32614/RJ-2018-009
Pictet, F.J., 1846, Traité élémentaire de paléontologie ou histoire naturelle des animaux fossiles, considérés dans leurs rapports zoologiques et géologiques: Paris, France, Langlois et Leclerq, 458 p. https://doi.org/10.5962/bhl.title.25238
Pictet, F.J., 1853, Traité de paléontologie ou histoire naturelle des animaux fossiles considérés dans leurs rapports zoologiques et géologiques: Paris, France, J. -B. Baillière, 110p.
Pictet, F.J., 1854, Traité de paléontologie ou histoire naturelle des animaux fossiles considérés dans leurs rapports zoologiques et géologiques, 2a ed: Paris, France, Chez J.-B. Bailliere, 727 p.https://doi.org/10.5962/bhl.title.13903
Pieper, R.J., Korpel, A., 1983, Image processing for extended depth of field: Applied Optics 22(10), 1449–1453. https://doi.org/10.1364/AO.22.001449
Polz, H., 1998, Schweglerella strobli gen.nov. sp.nov. (Crustacea: Isopoda: Sphaeromatidea), eine Meeres-Assel aus den Solnhofener Plattenkalken: Archaeopteryx 16, 19–28.
Preibisch, S., Saalfeld, S., Tomancak, P., 2009, Globally optimal stitching of tiled 3D microscopic image acquisitions: Bioinformatics 25(11), 1463–1465. https://doi.org/10.1093/bioinformatics/btp184
Racovitza, E.G., 1912, Cirolanides (Premiere serie). Biospeleologica 27: Archives de Zoologie Experimentale et Generale 10, 203–329.
Rathbun, M.J., 1935, Fossil Crustacea of the Atlantic and Gulf Coastal Plain: USA, Geological Society of America Special Papers 2, 160p.
Reiff, E., 1936, Isopoden aus dem Lias Delta (Amaltheenschichten) Schwabens: Paläonto- logische Zeitschrift 18(1–2), 49–90. https://doi.org/10.1007/BF03041710
Remeš, M., 1912, Urda moravica n. sp. z doggeru Chřibů: Časopis Moravského musea zemského 12, 173–177.
Rollmann, W., 1853, Zwei neue stereoskopische Methoden: Annalen der Physik 90(1), 186–187.
Rybakov, A.V., 1990, Bourdonia tridentata gen. n., sp. n. (Isopoda: Cabiropsidae) a hyperparasite of Bopyroides hippolytes Kroyer from the shrimp Pandalus borealis: Parazitologiia 24(5), 408–416.
Schädel, M., Perrichot, V., Haug, J., 2019, Exceptionally preserved cryptoniscium larvae - morphological details of rare isopod crustaceans from French Cretaceous Vendean amber: Palaeontologia Electronica 22.3.71, 1–46. https://doi.org/10.26879/977
Schädel, M., van Eldijk, T., Winkelhorst, H., Reumer, J.W.F., Haug, J.T., 2020, Triassic Isopoda – three new species from Central Europe shed light on the early diversity of the group: Bulletin of Geosciences 95(2), 145–166. https://doi.org/10.3140/bull.geosci.1773
Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J.-Y., White, D.J., Hartenstein, V., Eliceiri, K., Tomancak, P., Cardona, A., 2012, Fiji: an open-source platform for biological-image analysis: Nature Methods 9(7), 676–682. https://doi.org/10.1038/nmeth.2019
Schiødte, J.C., Meinert, F.W., 1881, Symbolæ ad Monographiam Cymotharum Crustaceorum Isopodum Familiæ 2. Anilocridae: Naturhistorisk Tidsskrift 3(13), 166p. https://doi.org/10.5962/bhl.title.10300
Schram, F.R., 1970, Isopod from the Pennsylvanian of Illinois: Science, 169(3948), 854–855. https://doi.org/10.1126/science.169.3948.854
Schram, F.R., 1974, Paleozoic Peracarida of North America: Fieldiana Geology 33(6), 95–124.
Schubert, R., 2000, Using a flatbed scanner as a stereoscopic near-field camera: IEEE Computer Graphics and Applications 20(2), 38–45. https://doi.org/10.1109/38.824535
Schultz, G.A., 1977, Bathypelagic isopod Crustacea from the Antarctic and southern Seas, in Schultz, G.A., (ed.), Biology of the Antarctic Seas V: Washington, D.C., American Geophysical Union, 69–128. https://doi.org/10.1029/AR023p0069
Schweigert, G., 2015, Zehnfußkrebse (Decapoda) und andere Krebstiere, in Arratia, G., Schultze, H.-P., Tischlinger, H., Viohl, G. (eds), Solnhofen – Ein Fenster in die Jurazeit: München, Germany, Pfeil, 271–291.
Seed, W.F., 1979, Article: The family Gnathiidae (Crustacea: Isopoda). A new Victorian species: The Victorian Naturalist, 96, 56–62.
Serrano-Sánchez, M. de L., Nagler, C., Haug, C., Haug, J.T., Centeno-García, E., Vega, F.J., 2016, The first fossil record of larval stages of parasitic isopods: cryptoniscus larvae preserved in Miocene amber: Neues Jahrbuch für Geologie und Paläontologie - Abhandlungen 279(1), 97–106. https://doi.org/10.1127/njgpa/2016/0543
Shiino, S.M., 1954, A new fresh-water entoniscid isopod, Entionella okayamaensis n. sp.: Reports of the Faculty of Fisheries, Prefectural University of Mie 1(3), 239–246.
Smit, N.J., Basson, L., Van As, J.G., 2003, Life cycle of the temporary fish parasite, Gnathia africana (Crustacea: Isopoda: Gnathiidae): Folia Parasitologica 50(2), 135–142. https://doi.org/10.14411/fp.2003.024
Smit, N.J., Bruce, N.L., Hadfield, K.A., 2014, Global diversity of fish parasitic isopod crustaceans of the family Cymothoidae: International Journal for Parasitology: Parasites and Wildlife 3(2), 188–197. https://doi.org/10.1016/j.ijppaw.2014.03.004
Smit, N.J., Van As, J.G., Basson, L., 1999, A redescription of the adult male and praniza of Gnathia africana Barnard, 1914 (Crustacea, Isopoda, Gnathiidae) from southern Africa: Folia Parasitologica 46, 229–240.
South, A., 2017, rnaturalearth: World Map Data from Natural Earth. R packages.
Stolley, E., 1910, Über zwei neue Isopoden aus norddeutschem Mesozoikum: Jahresberichte der Naturhistorischen Gesellschaft zu Hannover 60, 191–216.
Sutton, M.D., Rahman, I.A., Garwood, R.J., 2014, Techniques for virtual palaeontology, New analytical methods in earth and environmental science: Hoboken, NJ ; Chichester, West Sussex, Wiley Blackwell, 200 p.
Tanaka, K., 2005, A new genus and species of gnathiid isopod (Isopoda, Gnathiidae) from the Ryukyus, Southwestern Japan: Journal of Crustacean Biology, 25(4), 565–569. https://doi.org/10.1651/C-2605.1
Taylor, B., 1972, An urdidid isopod from the Lower Cretaceous of south-east Alexander Island: Bulletins of the British Antarctic Survey 27, 97–103.
Tennekes, M., 2018, tmap: Thematic Maps in R: Journal of Statistical Software 84(6), 1–39. https://doi.org/10.18637/jss.v084.i06
Thamban, A.P., Kappalli, S., Kottarathil, H.A., Gopinathan, A., Paul, T.J., 2015, Cymothoa frontalis, a cymothoid isopod parasitizing the belonid fish Strongylura strongylura from the Malabar Coast (Kerala, India): redescription, description, prevalence and life cycle: Zoological Studies 54, 42. https://doi.org/10.1186/s40555-015-0118-7
Thing, C.Y., Ota, Y., Hatai, K., Ransangan, J., 2015, Redescription of Caecognathia coralliophila (Monod, 1926) (Crustacea, Isopoda, Gnathiidae), collected from a fish hatchery in Sabah, Borneo Island, Malaysia: Proceedings of the Biological Society of Washington, 128(1), 51–62. https://doi.org/10.2988/0006-324X-128.1.51
Tischlinger, H., Arratia, G., 2013, Ultraviolet light as a tool for investigating Mesozoic fishes, with a focus on the ichthyofauna of the Solnhofen archipelago: Mesozoic fishes – Global Diversity and Evolution: München, Germany, Verlag Dr. Friedrich Pfeil, 549–560.
van der Wal, S., Haug, J.T., 2020, Shape of attachment structures in parasitic isopodan crustaceans: the influence of attachment site and ontogeny: PeerJ, 8, e9181. https://doi.org/10.7717/peerj.9181
van der Wal, S., Schädel, M., Ekrt, B., Haug, J.T., 2021, Description and ontogeny of a 40-million-year-old parasitic isopodan crustacean: Parvucymoides dvorakorum gen. et sp. nov.: PeerJ, 9(e1231), 1–46. https://doi.org/10.7717/peerj.12317
Van Straelen, V., 1928, Contribution a l’etude des Isopodes Méso- et Cénozoiques, in Mémoires de l’Académie Impériale et Royale des Sciences et Belles-Lettres de Bruxelles 9, 1-59.
Viohl, G., 1994, Fish taphonomy of the Solnhofen Plattenkalk – an approach to the reconstruction of the palaeoenvironment. Taphonomie des poissons des Calcaires en Plaquettes de Solnhofen – intérêt pour la reconstruction du paléoenvironnement: Geobios 27(Supplement 1), 81–90.
Wägele, J.-W., 1989, Evolution und phylogenetisches System der Isopoda: Stand der Forschung und neue Erkenntnisse, Zoologica: Stuttgart, Germany, Schweizerbart, 262 p.
Wägele, J.-W., Brandt, A., 1988, Protognathia n. gen. bathypelagica (Schultz, 1977) rediscovered in the Weddell Sea: A missing link between the Gnathiidae and the Cirolanidae (Crustacea, Isopoda): Polar Biology, 8(5), 359–365. https://doi.org/10.1007/BF00442027
Walther, J., 1904, Die Fauna der Solnhofener Plattenkalke. Bionomisch betrachtet: Festschrift zum siebzigsten Geburtstage von Ernst Haeckel: Jena, Germany, Gustav Fischer, 135–214. https://doi.org/10.5962/bhl.title.142760
Watling, L., 1981, An alternative phylogeny of peracarid Crustaceans: Journal of Crustacean Biology 1(2), 201–210. https://doi.org/10.2307/1548159
Wheatstone, C., 1838, On some remarkable, and hitherto unobserved, phenomena of binocular vision: Philosophical Transactions of the Royal Society of London 128, 371–394.
Wickham, H., 2007, Reshaping Data with the reshape Package: Journal of Statistical Software, 21(12),1-20. https://doi.org/10.18637/jss.v021.i12
Wickham, H., 2009, ggplot2: Elegant Graphics for Data Analysis, Use R!: New York, Springer, 212 p. https://doi.org/10.1007/978-0-387-98141-3
Wickham, H., François, R., Henry, L., Müller, K., 2020, dplyr: A Grammar of Data Manipulation: R. https://dplyr.tidyverse.org/
Wieder, R.W., Feldmann, R.M., 1989, Palaega goedertorum, a fossil isopod (Crustacea) from late Eocene to early Miocene rocks of Washington State: Journal of Paleontology, 63(01), 73–80. https://doi.org/10.1017/S0022336000040981
Wieder, R.W., Feldmann, R.M., 1992, Mesozoic and Cenozoic fossil isopods of North America: Journal of Paleontology, 66(6), 958–972. https://doi.org/10.1017/S0022336000021041
Wilke, C.O., 2020, ggtext: Improved Text Rendering Support for ggplot2: R.
Wilson, G.D., 1996, Of uropods and isopod crustacean trees: A comparison of “groundpattern” and cladistic methods: Vie et Milieu, 46(2), 139–154.
Wilson, G.D.F., 2003, A new genus of Tainisopidae fam. nov. (Crustacea: Isopoda) from the Pilbara, Western Australia: Zootaxa, 245(1), 1–20. https://doi.org/10.11646/zootaxa.245.1.1
Wilson, G.D.F., Sims, C.A., Grutter, A.S., 2011, Toward a taxonomy of the Gnathiidae (Isopoda) using juveniles: The external anatomy of Gnathia aureamaculosa zuphea stages using scanning electron microscopy: Journal of Crustacean Biology, 31(3), 509–522. https://doi.org/10.1651/10-3432.1
Wittler, F.A., 2007, Ein Isopode im Mittelbajocium von Velpe bei Osnabrück (Crustacea, Dogger, NW – Deutschland): Arbeitskreis Paläontologie Hannover 35(1), 15–21.
Zittel, K.A.von, 1885, Handbuch der Palaeontologie. 1 Abtheilung Palaeozoologie. II. Band – Monografien Geowissenschaften Gemischt : München, R. Oldenbourg, 893 p.
Zittel, K.A.von, 1887, Traité de Paléontologie. Tome II, Partie I. Mollusca et Arthropoda: Paris, France, Octave Doin, 897 p.
Peer Reviewing under the responsibility of Universidad Nacional Autónoma de México.
This is an open access article under the CC BY-NC-SA license(https://creativecommons.org/licenses/by-nc-sa/4.0/)

