|
Boletín de la Sociedad Geológica Mexicana Volumen 74, núm. 3, A140622, 2022 http://dx.doi.org/10.18268/BSGM2022v74n3a140622
|
 |
Environmental variation in the early and middle Holocene in Tequendama and Aguazuque archaeological sites, Colombia
Variación ambiental en los sitios arqueológicos del Holoceno temprano y medio de Tequendama y Aguazuque, Colombia
Angélica Viviana Triana Vega1,2,*, Víctor Adrián Pérez Crespo3
1 Externado University of Colombia, Faculty of Cultural Heritage Studies, Calle 12 No. 1-17 Este. Bogotá, Colombia.
2 University of the Andes, Department of Anthropology, Carrera 1 No. 18 A-10 – Piso 6, Bloque G -GB, 111711, Bogotá, Colombia.
3 National Autonomous University of Mexico, Institute of Geology, Circuito de la Investigación Científica, Ciudad Universitaria, Coyoacán, 04510, CDMX, Mexico.
* Corresponding author: (A. V. Triana Vega) This email address is being protected from spambots. You need JavaScript enabled to view it.
How to cite this article:
Triana Vega, A.V., Pérez Crespo, V.A., 2022, Environmental variation in the early and middle Holocene in Tequendama and Aguazuque archaeological sites, Colombia: Boletín de la Sociedad Geológica Mexicana, 74 (3), A140622. http://dx.doi.org/10.18268/BSGM2022v74n3a140622
Manuscript received: December 15, 2021; Corrected manuscript received: March 18, 2022; Manuscript accepted: Jun 23, 2022.
ABSTRACT
This study focuses on identifying possible environmental variations and plant availability during the occupation in two archaeological sites: Tequendama and Aguazuque, located in Sabana de Bogotá, Colombia. Those sites represent periods of occupation during the early to middle Holocene that contributes valuable information about hunter-gatherers who occupied this area. Data obtained and recovered archaeological material during excavations shed light on relatively continuous occupations until the late Holocene. The presence of lithic artefacts, human and fauna bone remains offered relevant information to comprehend social dynamics among these human groups; likewise, phytolith and stable isotope analysis on sediments and fauna were carried out to identify environmental variations, and the presence of plant remains in these archaeological contexts. Carbon isotope analysis in sediments indicated the prevalence of plants C3 from the early Holocene. In turn, isotopic relationships in carbon obtained from mammals’ dental enamel found in both sites suggest that those animals consumed such types of plants. Also, isotopic values in oxygen from dental enamel show humid and possibly cold environmental conditions in both locations. Also, phytolith analyses provide evidence on the types of plants available in determined contexts and reconstruct environments, use, and availability. Those three types of analysis were applied to archaeological contexts to determine the presence of plants type C3 or C4 available in the sites, which permitted to evidence of environmental changes, humid conditions, and, in a few cases, drought across occupation, as well as the differences in terms of the presence or absence of certain types of plants during chronological periods, suggesting a possible association of horticultural processes and domestication during middle Holocene in Aguazuque archaeological site.
Keywords: carbon stable isotopes, phytoliths, early and middle Holocene, hunter-gatherers, Sabana de Bogotá, Colombia.
RESUMEN
La presente investigación se concentró en identificar si existen variaciones ambientales y la disponibilidad de plantas durante la ocupación de los sitios arqueológicos de Tequendama y Aguazuque ubicados en la Sabana de Bogotá, Colombia. Dichos sitios representan periodos de ocupación durante el Holoceno temprano y medio, aportando una valiosa información acerca de los cazadores-recolectores que ocuparon este espacio. Los datos obtenidos y el material arqueológico recuperado durante las excavaciones dan cuenta de ocupaciones casi continuas hasta el Holoceno tardío inicial. La presencia de artefactos líticos, restos óseos humanos y de fauna aportaron información importante para ampliar las dinámicas sociales de estos grupos; así mismo, se contemplaron análisis especializados como los fitolitos e isótopos estables en sedimento y fauna que permitieran comprender las variaciones ambientales y la presencia de plantas en estos contextos arqueológicos. Los análisis isotópicos de carbono en los sedimentos indican que la prevalencia de plantas C3 desde el Holoceno temprano y en el caso de las relaciones isotópicas de carbono procedentes del esmalte de los mamíferos hallados en ambos sitios indican que estos animales consumían este tipo de plantas. Asimismo, los valores isotópicos de oxígeno del esmalte dental muestran condiciones húmedas y posiblemente frías en ambas localidades. Los análisis de fitolitos permiten evidenciar el tipo de plantas disponibles en determinados contextos y reconstruir ambientes, uso y disponibilidad de plantas. Estos tres análisis fueron aplicados a los contextos arqueológicos para determinar la presencia de plantas tipo C3 o C4 disponibles en los sitios, lo anterior, permitió evidenciar los cambios ambientales ocurridos a nivel de sitio, las condiciones de humedad o en algunos casos de sequía a lo largo de la ocupación y las diferencias existentes en la presencia o ausencia de cierto tipo de plantas por periodos cronológicos que sugiere quizá una asociación a procesos de horticultura y domesticación durante el Holoceno medio para el sitio de Aguauzuque.
Palabras clave: isótopos estables de carbono, fitolitos, Holoceno temprano y medio, cazadores-recolectores, Sabana de Bogotá, Colombia.
- Introduction
Understanding human occupation and the changes that may have occurred during the transition spanning between the end of the Pleistocene and the early and middle Holocene has been a subject of great relevance in archaeological studies. A particular aspect in those studies has been the identification of environmental conditions that somehow may have permitted the presence of the first settlers concerning the availability of resources and their optimization and even the development of specific activities such as horticulture and agriculture (Grayson, 1981; Reitz and Wing, 2008).
Aguazuque and Tequendama archaeological sites are in Sabana de Bogota (Figure 1). Aguazuque is an open-air archaeological site where the human occupation has been found from the late to middle Holocene. Human burials, lithic artefacts, animal and carbonized vegetal remains have commonly been associated with horticulture and domestication processes (Correal, 1990; Triana, 2019). In contrast, Tequendama is a rocky shelter dated between the ends of late Pleistocene until the beginnings of late Holocene, where human remains, lithic artefacts, animal and vegetal remains have been found. Tequendama is also relevant because it was one of the first sites where radiocarbon dating reported early Holocene in a human bone and because it shows a continuous occupation during Holocene (Correal and Van der Hammen, 1977; Triana, 2019). Both sites show specific characteristics, archaeological and geomorphological conditions that have allowed generating new hypotheses on the social dynamics that occurred in these two sites (Triana, 2019; Triana et al., 2019; Triana et al., 2020a; Triana et al., 2020b).
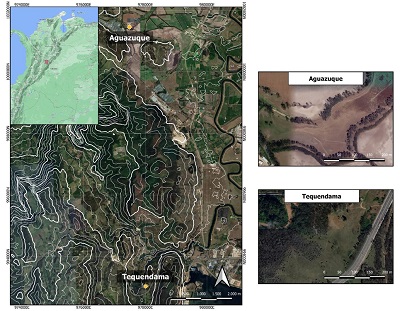 |
| Figure 1. Location of archaeological sites. |
Aguazuque and Tequendama have temperatures between 12 ºC and 18 ºC; the average annual precipitation is 698 mm; altitude at the sites ranges between 2.000 and 3.000 m. above sea level. Yet, Tequendama has an altitude of 2.570 meters above sea level and it is characterized by having a dry montane forest, while Aguazuque altitude is 2.550 and a lowland wet rainforest predominates (Correal, 1990; Correal and Van der Hammen, 1977).
Paleoenvironmental studies carried out in both sites have provided evidence of changes in the environmental conditions from late Pleistocene to middle Holocene. During the Interstadial periods of Guantiva (12.500 BP a 11.000 BP approximately), there is evidence of an increase in precipitations, water level, and temperature, which allowed the formation of swamps and wells. Additionally, the presence of families such as Adoxaceae, Aquifoliaceae, Betulaceae, Botryococcaceae, Cunoniaceae, Myricaceae, Myrtaceae, Rubiaceae and Sapindacea, as well as the genus Alnus, Dodonaea, Ilex, Hyperacium, Myrica, Spermacoce (or Borreria), Viburnum and Weimannia indicate the presence of swamps during the late Holocene and a decrease in temperatures in Sabana de Bogotá in a more general way (Correal, 1990; Van der Hammen, 1992).
Denser vegetation in warmer climates is represented during this period in families such as Myricaceae, Symplocaceae, Fagaceae, and Sapindaceae, and others reported that correspond to the Holocene and Late Glacial, which were also evidenced by pollen analyses (Van der Hammen, 1992). During the Abra stadial (11.000 BP), when a cold climate set in, open areas and subpáramo vegetation returned, and so did families such as Fagaceae and Asteraceae, as well as other new ones, as it is the case of Cyperaceae, Araliaceae o Apiaceae, Caryophyllaceae, and Salviniaceae. It is well known that climate started to improve progressively by the Holocene due to the rise in temperature, leading to swamp zones drying up and the growth of forest in front of El Abra.
Macroremains analyses in the Aguazuque archaeological site by Correal (1990) suggest that the climate in the middle Holocene, more specifically during the Hypsithermal period (6000 BP to 2500 BP, approximately), turned from humid to dry and, in certain seasons, it was warm, mild and cold. There is evidence showing that species such as Crescentia cujete (calabash tree), Cucurbita pepo (pumkin), Hieronyma macrocarpa (motilón), Ipomoea batatas (sweet potato), Oxalis tuberosa (yam), Persea americana (avocado) and Zea mays (corn) were domesticated.
From the 121 families reported, the following can be commonly found today in Sabana de Bogotá: Asteraceae, Ericaceae, Melastomataceae, Poaceae, Polygalaceae, Solanaceae, and Urticaceae. During the Holocene and Late Glacial, a cold climate has been reported, as well as predominantly open vegetation with families such as Caprifoliaceae, Gramineae or Poaceae, Plantaginaceae and Asteraceae, Melastomataceae, Urticaceae, Hyperacium, Betulaceae, Cunoniaceae, Chloranthaceae, and Sphagnaceae, which were identified through pollen analyses and radiocarbon dating (Van der Hammen, 1992).
The transition from the Pleistocene to Holocene brought about drier environmental conditions and a possible period of drought in Tequendama (Marchant, 2002; Mora and Pratt, 2002; Triana et al., 2020a). However, as late Holocene started, the climate was relatively warmer, and vegetation was made up of grasses and inundation areas. The results of carbon isotope relationships obtained by Cárdenas (2002), Delgado (2018), and Van der Hammen et al., (1990) from human remains in the area show values of ‒9‰ to ‒15 ‰, and they also provide information about the presence of plants type C4 and CAM plants during the various periods that compose the Holocene.
Meanwhile, the early Holocene gives evidence of plants C3 and later, during the middle Holocene, it is possible to trace changes in temperature, variations in climate, and fluctuations in precipitations, leading to more open and arboreal vegetation with a high frequency of grasses (Marchant, 2002; Mora and Pratt, 2002). This can be seen in isotope analyses applied to individuals in the area during that period, which reflect mixed diets C3/C4 (Cárdenas, 2002; Delgado, 2018; Triana, 2019; Triana et al., 2020b).
Nevertheless, most archaeological studies have focused on stable isotope analyses of human skeletal remains only, except for a few studies done on fauna bone remains by Martinez- Polanco (2016). Even if isotope values obtained from human bone remains are relevant to comprehend diet and ways of life in humans, it is also necessary to understand the environment, ecological availability of plants and animals, temperatures, and ecology in general in these two archaeological sites and during these periods to gain insights on the possible relationships among human-environment and articulate this data with social and ecological dynamics. That relationship will allow a joint vision of ecological availability and behavior in human beings in terms of diet and, more importantly, the consequences of the selection of certain resources and the behaviors related to this.
Thus, this paper intends to explore possible environmental conditions in Aguazuque and Tequendama by carrying out phytolith analyses, and carbon/oxygen isotope relationships from the dental remains of guinea pig (Cavia sp) and white-tail deer (Odocoileus virginianus) found in both sites, as well as δ13C values on soil samples of the sites with the purpose of contrasting this information with previous archaeological studies and then, contributing to our understanding of the environmental conditions that existed in Sabana de Bogotá from late Pleistocene to middle Holocene.
To do so, carbon isotope analysis on organic matter and isotopic values of carbon and oxygen in fauna dental enamel are performed, as compared to phytolith analyses, which constitute an innovative methodology applied to the archaeological sites in Sabana de Bogotá.
1.1. PHYTOLITH
Phytolith analysis has been widely applied because of its valuable paleo-ecological and paleoenvironmental information in the study of archaeological contexts (Piperno and Pearsall, 1998). Phytoliths are mineralized structures that formed due to the deposit of silica in vegetable cells and tissues, making a rigid, and quite often diagnostic, cast for cells. They show morphological features that fulfill certain functions, e.g., plants’ sustenance, resistance, or defense (Bertoldi de Pomar, 1975; Piperno and Pearsall, 1998; Twiss et al., 1969). Microremains can be deposited individually or as in groups. Likewise, an equal morphological type in different taxa, or the same taxon with morphological tissue variations, can deposit, which is commonly known as multiplicity and redundancy. Thus, phytolith analysis makes the identification of a specific part of a plant possible, contributing to the detection of structures where these micro components form and develop (Denham et al., 2007; Piperno and Pearsall, 1998). That is how phytoliths serve as a tool to identify several types of plants that can be found in paleo-ecological and archaeological contexts.
1.2. CARBON AND OXYGEN RATIOS IN HERBIVOROUS MAMMALS
There are three main forms of photosynthesis: C3, C4, and CAM (Smith and Epstein, 1971; Dawson et al., 2002; Andrade et al., 2007). The first, the C3 or Calvin-Benson cycle, is characteristic of trees and shrubs and some cold grasses and shows carbon isotope ratios of -23‰ to - 35‰ (Smith and Epstein, 1971; Ehleringer and Cerling 2002). In contrast, in the Hatch-Slack, or C4 cycle, its δ13C values, ranging from -12‰ to -14‰, are characteristic of grasses and some warm-zone trees and shrubs (Ehleringer and Cerling, 2002; Keeley and Rundel, 2003). Finally, the Crassulacean Acid Metabolism (CAM) pathway has δ13C values ranging from -12‰ to -35‰ and is found in succulent plants such as cacti, orchids, and bromeliads that cannot be distinguished from other plants by their photosynthetic pathway (Ehleringer and Cerling, 2002; Keeley and Rundel, 2003). Carbon is incorporated into herbivorous mammals when they feed on plants and their carbon isotopic values show an enrichment concerning that of plants, which will depend on the body mass and physiology of each species (Tejada-Lara et al., 2018).
As for oxygen, it enters mammals mainly through water that is consumed and, to a smaller degree, through the foods eaten and the air inhaled, which is balanced with the water lost by sweating, stool, urine, and exhalation (Koch, 2007). Such balance is controlled by the physiology of every organism and its body temperature (Sánchez, 2005). Since water is the main source of oxygen, its isotopic relationships are affected by geographical variables such as latitude, distance to the sea, altitude, and the amount of precipitation in a particular area, but mostly due to temperature (Dansgaard, 1964; Castillo et al. 1985; Mook, 2005). Besides, herbivores who obtain water from food show higher isotopic values than those who obtain oxygen by drinking water (Clementz et al., 2003). This explains why oxygen-isotopic relationships from herbivore dental enamel are commonly used in paleoclimatic studies to infer the presence of migration patterns (Koch, 2007).
1.3. CARBON RATIOS IN SOIL
Lehmann and Kleber (2015) believe that organic matter on soils is made up of vegetal and microbial biopolymers and that their products of degradation are in a constant state of biotic and abiotic decomposition. Both biotic and abiotic factors control decomposition rates, but those are affected by humidity and soil temperature, which can change saprotrophic microbial activity (Schmidt et al., 2011).
Carbon is incorporated into organic matter as a mineral fraction by assimilation of microbiota that takes in carbon in plants and passes to soil through respiration or humus (Cruz and Cruz et al., 2016). Guerrero and Berlanga (2000) consider that the fractionation of carbon isotopes is minimal during this process. However, there can be a certain degree of fractioning due to diverse soil formation processes (Wynn et al., 2006). Thus, carbon isotopic values for organic matter in soil are indicative of the relative abundance of plants with C3 or C4 metabolism, which depends on climatic conditions (Guerrero and Berlanga, 2000).
- Materials and Methods
Sediment samples used in this study were recovered from stratigraphic profiles in Tequendama and Aguazuque. In Tequendama, a 1 m x 1 m section was done with a depth of 1.75 m., and stratigraphic levels were identified, including the vegetal layer. Samples were taken from all levels for phytoliths and carbon stable isotopes (Table 1). In Aguazuque, 4 stratigraphic columns were excavated with dimensions 50 x 50 cm. However, the samples for this study correspond only to column 1 (145 cm deep and 8 stratigraphic levels), from which phytolith and a few carbon isotope samples were recovered (Table 1).
| Table 1. Stratigraphic levels at the Tequendama and Aguazuque sites for analysis of phytoliths and isotopes (Triana, 2019). |
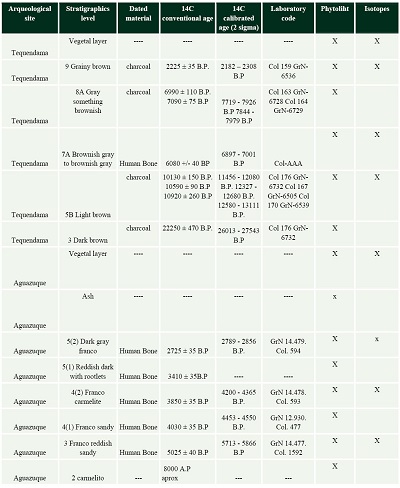 |
The results of phytolith analysis in the stratigraphic profiles of Tequendama and Aguazuque were obtained from the same profile used to carry out physical and chemical soil analyses.
Phytolith analysis was carried out as proposed in Piperno’s (2006) sample point methodology for each layer of excavation as follows: 200g of total sediment (soil) were collected from the stratigraphic profiles in Aguazuque and Tequendama every 5 cm following the next direction: northeast, northwest, southeast, southwest and grid center.
Later, 4 g of sediment were subjected to 25 ml of 7% hydrochloric acid. The sample was placed on a hot plate at 40 ºC until liquid reduced to 5 ml and was centrifuged at 1.000 rpm for 3 minutes. Next, the supernatant was removed, and the sample was filled up three times. A fraction of 15 ml 5% sodium hexametaphosphate was added, leaving it for a night. The sample was centrifuged three times more. 33% hydrogen peroxide was added while the mix was slightly shaken. It was left there for a night. The next day, the sample was centrifuged three times again. 10 ml sodium polytungstate of specific gravity (2,35 g/cm3) were added; the sample was shaken and centrifuged for three minutes at 1.000 rpm; The flotation fraction was recovered with an automatic pipette and later transferred to a new tube for a final series of centrifugation (three times). Methanol was added, followed by a final cycle of centrifugation (three times) (Madella et al., 1998).
The samples were set up on Canada-balsam-prepared slides so that the phytoliths could be rotated and better observed in 3d perspective. That allows a proper observation of details, such as ornamentation, shape, and corrosion or preservation indexes (Piperno, 2006; Posada, 2017). A count of 300 components per slide was later carried out, following international standards for phytolith counting (Madella et al., 2005). Those slides were observed through an Olympus BX51 petrographic microscope with 40X objective and with the assistance of Image-Pro Plus 7 software.
Phytolith taxonomic identification was based on the biomes recorded for the archaeological zone: low Andean Forest, flooded forests, and xerophytic dry zones (Calvachi, 2012; Pérez, 2000; Rangel, 2000). Thus, phytoliths classification is divided in four main ecological groups: 1) arboreal forest vegetation; 2) humid or swamp vegetation; 3) humid grass vegetation; 4) dry grass vegetation or xeric environments. Additionally, the taxonomy applied by Piperno (2006), Twiss et al. (1969) and Posada (2017) was followed as well.
2.1. STABLE ISOTOPE ANALYSIS
2.1.1. SAMPLE EXTRACTION AND DIAGENESIS TEST OF DENTAL ENAMEL
Three teeth of Odocoileus virginianus and two of Cavia sp were collected in 2014 in Aguazuque and Tequendama (see Table 2). To evaluate the degree of diagenesis, 10 mg were chosen, and each sample was ground and sieved (125 μm mesh size) to obtain a uniform fine powder. Later, these samples were analyzed by Fourier-transform infrared spectroscopy with attenuated total reflectance sampling (FT-IR-ATR) at Laboratorio de Geoquímica Ambiental Molecular, Instituto de Geología-UNAM, Laboratorio Nacional de Geoquímica y Mineralogía-Instituto de Geología (LANGEM). The FT-IR-ATR values of dental enamel were compared with the values of crystallinity index proposed by Weiner (2010) and Asscher et al. (2011), 2.5 to 4.6, to evaluate the presence of diagenesis.
| Table 2. FTIR, δ13C, δ13C Modern diet equivalent, δ18O18 and MAT values of dental enamel. |
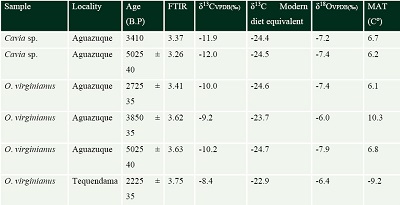 |
2.1.2. CARBON AND OXYGEN STABLE ISOTOPES FROM DENTAL ENAMEL
30 mg of dental enamel was pulverized and later prepared following the method proposed by Koch et al. (1997). First, 10 ml of 30% hydrogen peroxide were added and left for 2h to remove organic matter. The samples were centrifuged, and after hydrogen peroxide decantation, they were washed three times with Type 1 water (HPLC grade, 18.2 MΩ-cm). After washing, 5 ml of Ca(CH3CO2)2-CH3COOH 1.0 M, pH 4.75 buffer solution were added, and the mixture left to rest for 9h. The buffer solution was decanted, and the samples were washed another three times with Type 1 water. Finally, ethanol was added to remove any remaining water and the solution was left for 20 h in an oven at 90°C.
Oxygen and carbon isotopic ratios were determined with a Finnigan MAT 253 mass spectrometer at the laboratory1 with a dual inlet system, auxiliary GasBench equipment, with a GC Pal autosampler and a temperature-controlled aluminum plate adjoined to the mass spectrometer (Révész and Landwehr, 2002). Results were reported as δ18OVPDB and δ13CVPDB, normalized using NBS-19, NBS-18, and LSVEC to the Vienna-PeeDee Belemnite (VPDB) scale following the corrections described by Coplen (1988), Werner and Brand (2001), and Coplen et al. (2006). For this technique, the relative standard deviation was 0.2‰ for oxygen and carbon isotopic analyses (Brand et al., 2014).
1 Laboratorio de Isótopos Estables- LANGEM-UNAM.
2.1.3. CARBON AND OXYGEN STABLE ISOTOPES FROM ORGANIC MATTER OF SOILS
Four samples of soil from Tequendama and four from Aguazuque were collected, and later ground in an agate mortar to a fine powder so that thier organic carbon content could be evaluated. Later, the samples were decarbonated with 5 M HCl and 1 g of each of the samples were sent to Laboratorio de Isótopos Estables- LANGEM-UNAM where they were analyzed using a Finnigan MAT 253 mass spectrometer. Results were reported as δ13CVPDB, normalized using NBS-19, NBS-18, and LSVEC to the Vienna-PeeDee Belemnite (VPDB) scale following the corrections described by Coplen (1988), Werner and Brand (2001), and Coplen et al. (2006). For this technique, the relative standard deviation was 0.2‰ for carbon isotopic analyses (Brand et al. 2014).
2.1.4. STATISTICAL ANALYSES
The FT-IR-ATR values in dental enamel were compared with those (2.5 to 4.6) proposed by Weiner (2010) and Asscher et al., (2011) to evaluate the presence of diagenesis. To estimate carbon isotopic ratios, body mass values for Cavia sp. and O. virginianus were obtained from Gallina et al., (2010) and ADW (2021) to be used to calculate the fractionation factor for each species using the formula proposed by Tejada-Lara et al., (2018). With this, the modern equivalent of diet composition was estimated using the equation developed by Kohn (2010) and modified by Domingo et al. (2013):
δ13Cmodern diet = δ13Cleaf + δ13Cmodern atmosCO2 – δ13Cancient atmosCO2.where δ13Cleaf = δ13Ctooth-enrichment−14.1, expressed in ‰. The δ13Cmodern atmosCO2 is – 8‰ and δ13Cancient atmos CO2 is −6.5‰, value calculated by Tipple et al., (2010) for the pre-Industrial age.
To compare carbon isotopic ratios of dental enamel with that in soils, δ13C values of animals were subtracted with enrichment value proposed by Cerling and Harris (1999).
In the case of δ18O values, these were first converted to VSMOW using the formulas proposed by Faure (1977): δ18OVSMOW: 01.030901 * δ18OVPDB + 30.91, and then δ18O of water through the Iacumin et al. (1996) formula: δ18Owater = δ18OVSMOW – 33.63/0.998. Finally, the δ18Owater were converted to MAT (Mean Annual Temperature) using the formula of Rozanski et al. (1993): MAT(◦C)=δ18OH2O(VSMOW) + 12.68 /0.36.
- Results
3.1. PHYTOLITHS IN SEDIMENTS
3.1.1. STRATIGRAPHIC PROFILE OF TEQUENDAMA
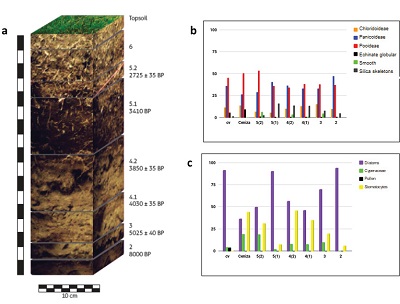
Tequendama profile is represented mostly by phytoliths of the Poaceae family and Pooideae, Panicoideae and Chloridoideae subfamilies. During observation, bullate and elongated phytoliths could be seen, which were not counted due to their high frequency in all slides. 300 components were identified in each slide, including phytoliths and others such as diatoms, pollen, stomatocysts, etc.
Bilobate roundel type, as well as crossed trapezoidal phytoliths belong to Pooideae and Panicoideae subfamilies. They are short cells that form in the plants’ epidermis and whose photosynthesis is C3 type. Those plants belong to open vegetation of grass, but their biome varies. For instance, Pooideae are typical of humid and mild climates; Ciperaceae also belong to this type of environment. On the other hand, Panicoideae are common in mild sub-humid environments, while Chloridoideae plants saddle type has a C4 photosynthesis and belong to drier, warmer xerophytic climates.
Most types of grass are indicators of environmental conditions since bullate phytoliths show common savannah-like grasses, while elongated cylindrical psylates do not have a particular taxonomic value and, thus, they are not environmental indicators. On the other hand, there are flat smooth rounded phytoliths belonging to a semi-open vegetation made of bushes and trees. Echinate globular phytoliths are associated to diagnostic identification in palm trees in general. Silica skeletons or articulated phytoliths are long cells of Poaceae that deposit in fossilized sections of epidermal tissue.
Diatoms, stomatocysts, and Ciperaceae phytoliths indicate humid and swamp-like conditions. Trichomes are mesophilic cells from where phytoliths detach and have the shape of hair or of the trichome base they separated from. Those components were counted despite not having been plotted due to the frequency they tend to appear. A brief description of the collected phytoliths will be provided next for each stratigraphic level (See Figures 2a, 2b and 2c to understand their distribution):
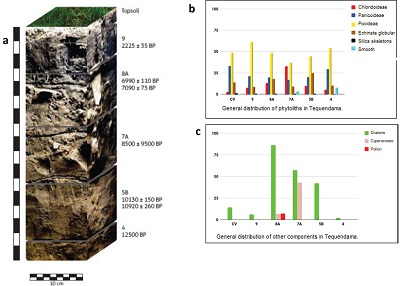 |
| Figure 2. Distribution of phytoliths in sediment stratigraphic profile of Tequendama. A) Stratigraphic profile, B) General distribution of phytoliths in Tequendama,C) General distribution of other components in Tequendama. |
- Vegetal layer:phytoliths abundance is high in this level, especially for those Pooideae, Panicoideae and echinate globular ones. There is an abundance of long cells, some of them are dendritic (Figure 3a) and diatoms. Silica skeletons and articulated phytoliths were also found in this level. This type of phytoliths is present in almost all levels, but their presence remains higher in the vegetal one. However, their frequency of appearance is quite low. A platelet phytolith was also observed that is associated to Asteraceae family (Figure 3b).
- Stratigraphic level 9: this level shows a great amount of bullate phytoliths and elongated cells of varied sizes: small, medium, and large, some of them surpass the 35 micra. Certain bullate phytoliths show corrosion. There is abundance of phytoliths in this level, most of them belonging to Pooideae, Panicoideae, echinate globular (Figure 3c) y Chloridoideae. Abundant organic material could also be seen, which may be related to the fact that this is vegetal layer. Trichomes appear on a low presence in this level too.
- Stratigraphic level 8A: in this level, bullate phytoliths and elongated cells can also be observed. Some bullates evidence corrosion, and trichomes have low frequencies. Phytoliths in this level are smaller in relation to the previous one, however, their frequency is high. There is evidence of organic matter, but it is not abundant. There is abundance of phytoliths’ fragments as well. Echinate globular show smaller sizes in this slide. Also, a variety of pollen, diatoms, and an increase of palm trees can be seen, in comparison to the previous level. Phytoliths are Pooideae and Panicoideae types, (Figure 3d), being the most frequent.
- Stratigraphic level 7A: this level shows a high frequency in the appearance of phytoliths. Most bullate phytoliths are broken but without corrosion. In contrast to previous levels, phytoliths presence here is not so high. Little organic matter and plenty of Panicoideae and Chloridoideae phytoliths (Figure 3e) can be observed. Moreover, several fragments of phytoliths were identified. It must be said that this level is the one with the highest quantity in the entire stratigraphic profile. Trichones are very abundant too. Smooth type phytoliths start to appear and echinate globular continue to show, although in a lower proportion if compared to previous levels. Trichomes can be observed from level 9, but their frequency of appearance is higher; these components were not plotted. Diatoms and Ciperaceae (Figure 3f) are also present in this level.
- Stratigraphic level 5B: there is evidence of carbonized organic matter, although it is not abundant. The abundance and variety of phytoliths reduced considerably in comparison to the previous level. The presence of bullate phytoliths and elongated cells show low frequencies and corrosion in some cases. The abundance of diatoms, Pooideae phytolith type, followed by Panicoideae and Chloridoideae is evident in this level. This level has the highest presence of Echinate globular phytoliths and trichomes, but they decrease slightly in this level.
- Stratigraphic level 4: elongated and bullate phytoliths are not so abundant in this level and some of them show corrosion. Fragments of phytoliths and organic matter are not abundant in this level. Yet, there are Pooideae type phytoliths with variations in their size: some are small, 4,8 micra, and some others quite large, 19 micra, approximately; Nevertheless, smaller phytoliths are very abundant. Smooth type phytoliths show a high frequency of appearance in this level. Additionally, a phytoliths associated to Aristidae and Poaceae families was observed, which indicates milder to warmer climates.
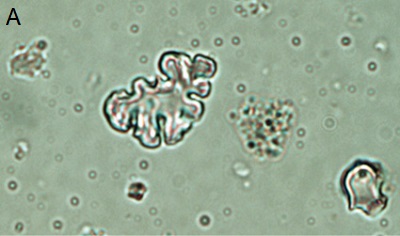 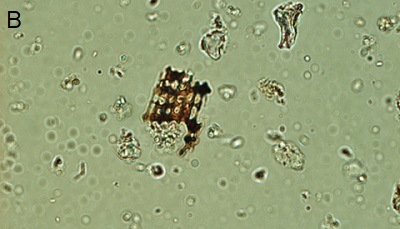 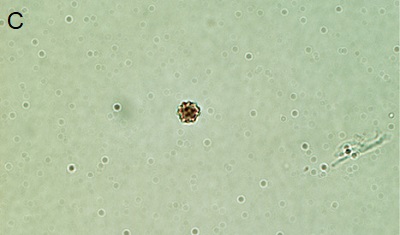 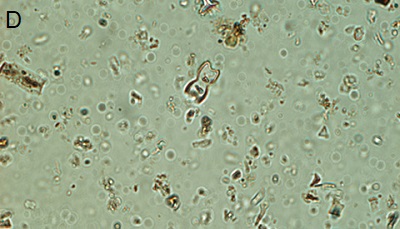 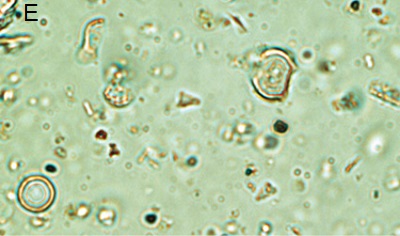 |
| Figure 3. Phytoliths present in Tequendama. A) Denditric phytolith, B) Platelet-type phytolith, C) equinate globular phytolith, D) panicoid-type phytolith, E) phytolith chloridoid (right) and diatom (left), F) Cyperaceae. All components were observed at 40X target. |
3.1.2. STRATIGRAPHIC PROFILE IN AGUAZUQUE
This stratigraphic profile is represented by the same frequent phytoliths in Tequendama, yet in a lower proportion. Pooideae remain the most frequent. Panicoideae phytoliths values increase in Aguazuque in comparison to Tequendama. Chloridoideae shows a lower abundance in this site. The frequency of appearance of components such as silica skeletons is higher and more abundant tan in Tequendama. Likewise, the appearance of components such as stomatocysts is observed only at this site. Observations per stratigraphic level are explained below (See the distribution for this profile in figures 4a, 4b and 4c).
 |
| Figure 4. Distribution of phytoliths in sediment stratigraphic profile of Aguazuque. A) Stratigraphic profile, B) General distribution of phytoliths in Aguazuque, C) General distribution of other components in Aguazuque. |
- Vegetal layer: The frequency of appearance of phytoliths in this level is high, but fragments of phytoliths makes its counting difficult. Large aggregates of organic matter can also be found there. Some phytoliths such as bullate and elongated cells show corrosion. Pollen grains were found, which is standard in a vegetal layer.
- Ash stratigraphic level: there is abundance of phytoliths, however, their fragments can also be found. The presence of elongated cells and bullates is high and some of them show corrosion. In general, they tend to be large. The presence of stomatocyst (Figure 5a) is abundant and begins in this level; Echinate globular phytoliths shows the highest presence in this level of the profile. Ciperaceae’s presence is higher here than in Tequendama.
- Stratigraphic level 52: there is abundance of phytoliths and their fragments. However, their presence deacreases if compared to previous levels. The amount of bullates decreased too in relation to the frequency of appearance in Tequendama. Elongated cells are still present in the site and level. Little organic matter could be seen, but there is a rise in smooth type phytoliths (Figure 5b) as well as in silica skeletons (Figure 5c). On the other hand, echinate globular and trichomes start to decrease in frequency; Ciperaceae and stomatocysts maintained high values in this level.
- Stratigraphic level 51: this sample shows components that had not been noticed before. Even though phytoliths can be seen, abundance is relatively low, as it has been noted here. On the contrary, the presence of silica skeletons is quite high in the slide and diatoms are very frequent with, at least, three diverse types that were observed. Elongated phytoliths are frequent and of many sizes. Bullates could also be observed, although in a lower number with cases of corrosion. Components such as stomatocysts and Ciperaceae decreased in this level.
- Stratigraphic level 42: there are several fragments of Phytoliths, a few bullates, but many elongated cells. A vast variety of components can be observed here, especially plenty of silica skeletons as it is the case of level 52. Yet, this sample shows the highest abundance. The presence of diatoms remains high, but not as much as in the previous level. There are at least three distinct types of diatoms as in level 52. Stomatocysts and Ciperaceae increase again in this level, as well as smooth phytoliths. Fragments of tracheids were observed (Figure 5d), which indicate arboreal environments; there are three phytoliths associated to Aristidae family (Figure 5e), which are indicators of mild to warm climates. Echinate globular phytoliths reduced considerably in earlier levels. In general, this slide shows more varied components for Aguazuque.
- Stratigraphic level 41: there is hardly any evidence of organic matter, yet plenty of phytoliths and their fragments. Several silica skeletons can also be observed, which were also found in levels 52 and 42. Stomatocysts and diatoms decrease in this sample. Ciperaceae are still present. Some bullate phytoliths show corrosion and a Bambosoideae phytolith was identified (Figure 5f). Trichomes, elongated cells and echinate globular phytoliths have low frequencies.
- Stratigraphic level 3: very scattered and fragmented phytoliths were observed in this level, as well as evidence of organic matter. Bullates and elongated cells were found too in a lower frequency in comparison to levels 51, 42and 41; some of these phytoliths show corrosion. It is important to mention that Chloridoideae and Panicoideae increase their abundance in deeper levels. Chloridoideae show the highest presence at this level. Smooth phytoliths, diatoms and Ciperaceae increase once again. On the other hand, Pooideae decreased in relation to the latest levels, the same for stomatocysts and silica skeletons.
- Stratigraphic level 2: Several components can be observed here. There is evidence of much organic matter and fragments of phytoliths, which are often occluded or are hard to be seen. Large bullate and elongated cells are not present any longer. Yet, there are short elongated cells measuring 10 micra or less. Diatoms are abundant, but the variety of components that were observed in other levels are absent here. In the previous level, components were scattered and there was Little organic matter, which contradicts what occurs in this sample. Panicoideae phytoliths are the highest in this level, in turn, phytoliths types Pooideae, Chloridoideae, echinate globular, silica skeletons, smooth, stomatocysts and Ciperaceae decreased considerably in this last level.
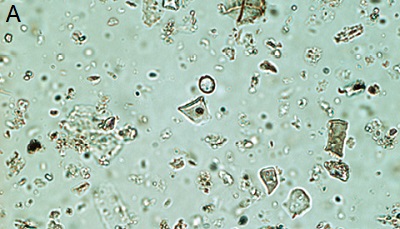
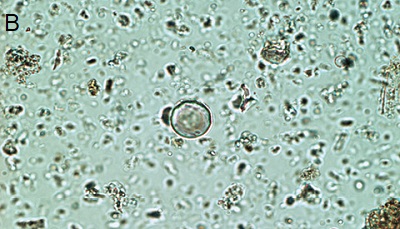
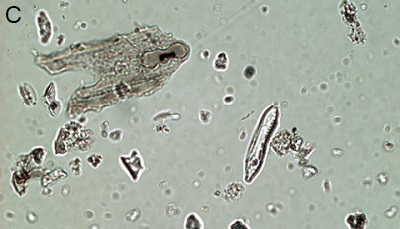
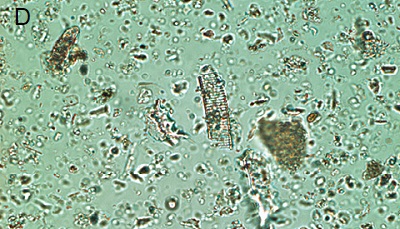
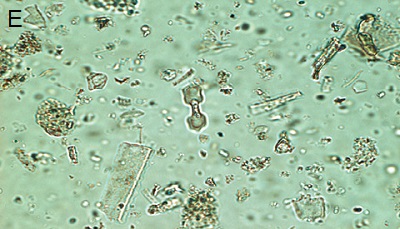
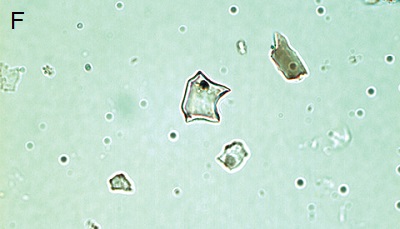
Figure 5. Phytoliths present in Aguazuque. A) Phytolith type pooid (iziquerda) and diatom (right), B) Smooth type phytolith, C) Silica skeleton (left top) and diatom (right bottom), D) Tracheida, E) Fitolito aristidae, F) Bambusoid phytolith.
The phytoliths observed in Aguazuque exhibit combined vegetation between C3 and C4 plants. Palm trees were included, but their presence is not as high as in Tequendama. Plants associated to arboreal vegetation were seen in some levels of occupation. Nevertheless, there are some differences in the types of plants in Aguazuque if compared to those in Tequendama. To illustrate, silica skeletons (articulated phytoliths) and algae stomatocysts (associated to humid conditions) are not shown in Tequendama. On the other hand, higher percentages of Ciperaceae and diatoms were observed in Aguazuque.
3.2. RESULTS OF ISOTOPIC ANALYSIS OF CARBON IN ENAMEL AND SEDIMENT
Carbon isotopic average value in animals is -10.3‰ with a maximum value of -8.4‰, corresponding to deer in Tequendama, and a minimum value of -12.0‰ for a guinea pig in Aguazuque (table 1). As for organic matter in soil, maximum value is -21.1‰ (TEQ 037) and minimum -24.8‰ (TEQ 052) with an average of –22.7‰ (Table 3).
| Table 3. Carbon isotopic ratios of organic matter. |
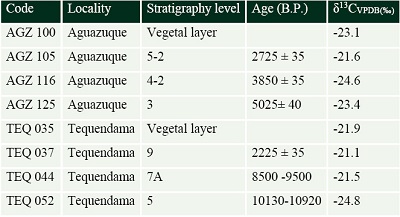 |
Figure 6 shows that guinea pigs show similar carbon isotopic values, even if they belong to different temporalities. On the contrary, deer change in time with values from -24.3‰ in 5025±40 B. P. for Aguazuque to -23.3‰ in 3085±35 B. P; those values decrease later as shown: -24.1‰ in 2773±35 B. P to arrive to -22.5‰ in 2225 ±35 B. P for Tequendama.
 |
| Figure 6. Comparison between carbon isotopic ratios of mammals and geological age. To δ13C values were added to enrichment factor of 14.1‰. |
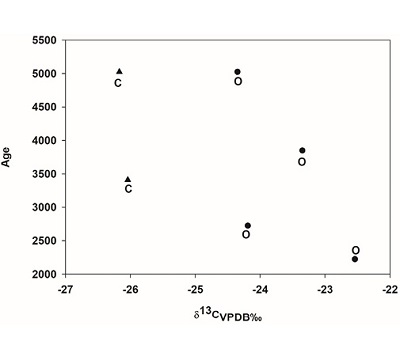
It can be observed in figure 7 that the most negative value -24.8‰ in soil organic matter comes from sample TEQ 052 in 10130, as compared to -21.5‰ in sample TEQ 044. Later, values go down to -23.4‰ in sample AGZ 125, and increase in sample AGZ 105, -21.6‰. Values in sample TEQ 037 increase to -21.5‰ as it is the case of samples TEQ 035 and AGZ 100, which correspond to current layers of organic matter in soil, as follows: -21.9‰ and -23.1‰.
 |
| Figure 7. Carbon isotopic ratios of organic matter from soil. |
3.3. RESULTS OF ISOTOPIC ANALYSIS OF OXYGEN IN ENAMEL
The average value of oxygen is δ18O -7.1 ‰ with the highest value being –6.0 represented in a deer in Aguazuque. In contrast, a deer in the same locality shows the lowest level, -7.9 ‰ (table 3). Moreover, oxygen isotopic levels in enamel show that guinea pig and deer dated 5025 ±40 B. P. have values of δ18O -7.4‰ and -7.9‰. Then, oxygen isotopic value for 3850 ±35 B. P. decreases to –9.2‰ and later increases up to 7.4‰ in 2725 ±35 B.P. Finally, these values conclude in –6.4‰ in 2225 ±35 B. P. (Figure 8).
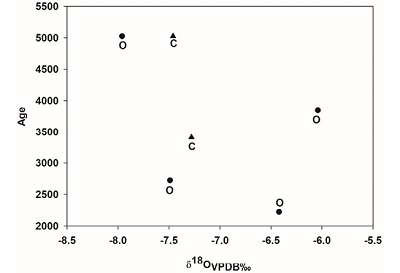 |
| Figure 8. Comparison between oxygen isotopic ratios of mammals and geological age. |
- Discussion
Data obtained from phytoliths in Tequendama during the transition between Pleistocene to early Holocene coincide with micro-morphological and physical-chemical analyses carried out by Triana (2019) in the same stratigraphic levels for this locality, which show humid environmental conditions in the end of Pleistocene and beginnings of Holocene. That can also be observed in carbon isotopic relationships in soil organic matter collected from level 5B in the site.
Subsequently, phytolith analyses reveal abundance in palm trees during the middle Holocene, more specifically C3 plants in levels 7A and 8A, as well as plants associated to humid conditions such as diatoms and Ciperaceae. Those correspond to a period of climate warming during the middle Holocene that is also the moment of higher archaeological occupation in Tequendama (Delgado, 2018, 2017, 2012; Marchant, 2002; Mora and Pratt, 2002; Van der Hammen, 1992). The recovered phytoliths show the presence of plants such as Pooideae (C3), Chloridoideae and Panicoideae (C4). Also, expressed in the values δ13C of organic matter in soil from both levels, there is evidence of mixed vegetation C3/C4, with predominance in C3 plants.
Moreover, the presence of diatoms and Ciperaceae in these two levels can be indicators of humid conditions, possibly associated to occasional flooding or strong anthropic activity recorded for the levels. Triana et al. (2019) state that micro-morphological and physical-chemical analyses in soil showed a relevant amount of bone fragments, often burnt; many sizes of charcoal fragments, some of them relatively large, and a clear transformation in soils. Also, this level shows the maximum magnetic susceptibility and dependence of frequency, which is a feature related to burning produced by anthropic activities. Additionally, a female individual burial was found, associated to high concentrations of animal bone remains such as deer and armadillo as well as lithic artifacts, among which scrapers and lithic debitage.
As for the transition to the end of middle Holocene, the presence of phytoliths associated to C3 grasses was observed, however, carbon isotopic relationships in level 9 show the presence of a mixed vegetation C3/C4 where the former dominates. There is also presence of Chloridoideae plants in this level with a low frequency of appearance if compared to C3 plants. Nowadays, the vegetation in the zone is dominated by C3 plants, which can be seen in modern organic matter.
In contrast, Aguazuque shows high concentrations of silica skeleton phytoliths, the most commonly corresponding to Chloridoideae and Panicoideae subfamilies (C4). This shows that the environmental conditions at that time were warm, with fluctuations in precipitation, and there was open area vegetation as well as forests. Nevertheless, organic matter suggests that the most predominant type of vegetation was C3 plants as can be seen in level 3.
During the span of time ranging from 4.000 to 3.000 B.P., a high presence of phytoliths and components related to humid conditions was seen. That is the case of Ciperaceae belonging to swamp environments, and diatoms and stomatocysts associated to bodies of water that are absent in Tequendama. Considering that humidity is one of the factors that contribute to the presence of C3 plants, the conditions in this locality at that time favored the development of this type of plant, which can be seen in carbon isotopic analysis of levels 4 to 2 in Aguazuque (Medrano and Flexas, 2001).
Organic matter found in stratigraphic levels 5 to 2 show the presence of C3/C4 vegetation with dominance in the former. That is consistent with the presence of smooth type phytoliths associated to arboreal vegetation, and plants linked to Arecaceae family such as palm trees. Besides, the presence of components associated to humid conditions such as Ciperaceae and stomatocysts was seen.
In terms of carbon isotopic values obtained from guinea pig, it is possible to say that these animals mostly consumed C3 plants. Similarly, deer consumed those plants as well, which is in concordance with the results obtained in phytoliths and organic matter from both archaeological sites and shows the dominance of those plants in Tequendama and Aguazuque during the late Pleistocene to today.
In turn, oxygen isotopic values in animals suggest that those probably drank water from the same sources; the yearly average temperature obtained from oxygen isotopic relationships in guinea pig and deer dental enamel show that temperatures during middle Holocene ranged between 6 to 12, lower than today’s 12º C a 18º C. Deer recovered in Aguazuque and dated 3850 ± 35 provides evidence of temperatures lower between 2 to 8 degrees than today, indicating colder conditions that coincide with records of phytoliths.
Finally, isotopic relationships in Tequendama from deer dated 2225 ± 35 show that temperatures were between 3 to 9 degrees below current temperatures in the area. One of the factors that control C3 and C4 plant distribution is temperature, since temperatures below 25ºC promote the development of C3 plants and, in contrast, C4 plants decrease (Medrano and Flexas, 2001). Therefore, the temperatures recorded in oxygen isotopic relationships from animals explain the predominance of C3 plants, as it was seen in carbon isotopic values from organic matter collected in both sites.
- Conclusions
The results derived from the analyses carried out in this study suggest that the environmental conditions during the transition between Holocene and the period of occupation in Tequendama and Aguazuque archaeological sites show a dominance of C3 plants across the occupation, suggesting possible plant processing or the beginnings of horticulture –as it has been proposed for Aguazuque before- which may have included C3 plants such as tubers. Also, in the scenario that plants of C4 photosynthesis had existed, they may not have completely deposited yet.
However, the variations in organic matter shown in the isotopic results do not show strong changes or variability that prove the presence of C4 plants in any of the two archaeological sites during the whole occupation.
Finally, it is necessary to complement these stable isotopes studies in the future by widening fauna and flora isotopic ecology (both modern and archaeological) and sampling other bodies and sources of water in Sabana de Bogotá that allows us to understand environmental changes or patterns of mobility in these two archaeological sites during early and middle Holocene. About phytoliths, collections of reference can be used to deepen and widen specific information for each archaeological site.
Contributions of authors
(1) Conceptualization: AVTV and VAPC; (2) Analysis or data acquisition: AVTV and VAPC; (3) Methodologic/technical development: AVTV and VAPC; (4) Writing of the original manuscript: AVTV and VAPC; (5) Writing of the corrected and edited manuscript: AVTV and VAPC; (6) Graphic design: AVTV and VAPC; (7) Fieldwork: AVTV and VAPC; (8) Interpretation: AVTV and VAPC; (9) Financing: institutional.
Financing
Fundación de investigaciones arqueológicas Banco de la República de Colombia (FIAN).
Acknowledgments
We would like to express our gratitude to all the professionals, laboratories, institutions and people who contributed directly or indirectly to this research. Thanks to Laboratorios de Isótopos Estables -LANGEM at UNAM, as well as to Pedro Botero, Isabel Casar, Edith Cienfuegos Alvarado, Francisco J. Otero Trujano y Rafael Puente M., for their analytical aid. To doctors Emily McLung and Cristina Adriano for the processing, identification and interpretation of phytoliths at Laboratorio de Paleoetnobotánica y Paleoambiente, Instituto de Investigaciones Antropológicas, Universidad Nacional Autónoma de México. We want to thank too doctors Elizabeth Solleiro and Sergey Sedov for their theoretical and methodological contributions. Our gratitude to Fundación de Investigaciones Arqueológicas (FIAN) for the support and funding. Finally, the landowners of Hacienda Tequendama and Hacienda Fute for their everlasting support, company and permissions to continue working in such wonderful archaeological sites, as well as for their true interest and respect for archaeology as a discipline and their encouragement to keep going with our research in Tequendama and Aguazuque.
Conflicts of interest
No potential conflict of interest was reported by the authors.
References
Animal Diversity Web (ADW) (2021, Online database and encyclopedia of animal natural history: USA, University of Michigasn, Museum of Zoology. https://animaldiversity.org/
Andrade, J.L., De La Barrera, E., Reyes-Garcia, C., Ricalde, M.F., Vargas-Soto, G., Cervera, C.J., 2007, El metabolismo ácido de las crasuláceas: diversidad, fisiología ambiental y productividad: Boletín de la Sociedad Botánica de México, 87, 37–50.http://dx.doi.org/10.17129/botsci.1764
Asscher, Y., Regev, L., Weiner, S., Boaretto, E., 2011, Atomic disorder in fossil tooth and bone mineral: an FTIR study using the grinding curve method: ArcheoSciences, revue d’Archéométrie, 35(35),135–141. https://doi.org/10.4000/archeosciences.3062
Bertoldi de Pomar, H., 1975, Los silicofitolitos: sinopsis de su conocimiento: Revista Darwiniana, 19 (2-4), 173-206.
Brand, W. A., Coplen, T. B., Vogl, J., Rosner, M., Prohaska, T., 2014, Assessment of international reference materials for isotope-ratio analysis (IUPAC Technical Report): Pure and Applied Chemistry, 86(3), 425–467. https://doi.org/10.1515/pac-2013-1023
Calvachi, B., 2012, Los ecosistemas semisecos del altiplano cundiboyacense, bioma azonal singular de Colombia, en gran riesgo de desaparición: Revista Mutis, 2 (2), 26-59. https://doi.org/10.21789/22561498.364
Castillo, R., Morales, P., Ramos, S., 1985, El oxígeno-18 en las aguas meteóricas de México: Revista Mexicana de Física, 31(4), 637–647.
Cárdenas, F., 2002, Datos sobre la alimentación prehispánica en la Sabana de Bogotá, Colombia: Bogotá, Instituto Colombiano de Antropología e Historia, 71p.
Cerling, T.E., Harris, J.M., 1999, Carbon isotope fractionation between diet and bioapatite in ungulate mammals and implications for ecological and paleoecological studies: Oecologia, 120, 347-336. https://doi.org/10.1007/s004420050868
Clementz, M. T., Hoppe, K. A., Koch, P. L., 2003, A paleoecological paradox: the habitat and dietary preferences of the extinct tethythere Desmostylus, inferred from stable isotope analysis: Paleobiology, 29(4), 506-519. https://doi.org/10.1666/0094-8373(2003)029<0506:APPTHA>2.0.CO;2
Coplen, T., Brand, W.A., Gehre, M., Gröning, M., Meijer, H.A.J., Toman, B., Rm, V., 2006., New Guidelines for δ13C Measurements: Analytical Chemistry, 78(7), 2439–2441. https://doi.org/10.1021/ac052027c
Coplen, T.B., 1988, Normalization of oxygen and hydrogen isotope data: Chemical Geology: Isotope Geoscience section, 72(4), 293–297. https://doi.org/10.1016/0168-9622(88)90042-5
Correal, G., Van der Hammen, T., 1977, Investigaciones arqueológicas en los abrigos rocosos del Tequendama: 12.000 Años de historia del hombre y su medio ambiente en la altiplanicie de Bogotá: Colombia, Bogotá, Fondo de Promoción de la Cultura del Banco Popular, 194p.
Correal, G., 1990, Aguazuque: evidencias de cazadores, recolectores y plantadores en la altiplanicie de la Cordillera Oriental: Colombia, Bogotá, Fundación de Investigaciones Arqueológicas Nacionales, Banco de la República, 316p.
Cruz-y-Cruz, T., Pérez-Crespo, V.A., Pustovoytov, K., Sedov, S., Morales-Puente, P., Tovar-Liceaga, R.E., Arroyo Cabrales, J., Terrazas-Mata, A., Sánchez-Miranda, G., 2016, Paleosol (organic matter and pedogenic carbonates) and paleontological δ13C records applied to the paleoecology of Late Pleistocene-Holocene in Mexico: Quaternary International, 418,147-164. https://doi.org/10.1016/j.quaint.2015.12.093
Dansgaard, W., 1964, Stable isotopes in precipitation: Tellus, 16(4), 436–468. https://doi.org/10.1111/j.2153-3490.1964.tb00181.x.
Dawson, T.E., Mambelli, S., Plamboeck, A.H., Templer, P.H., Tu, K.P., 2002, Stable isotopes in plant ecology: Annual Review of Ecology and Systematics, 3(1), 507–559. https://doi.org/10.1146/annurev.ecolsys.33.020602.095451
Delgado, M., 2018, Stable isotope evidence for dietary and cultural change over the Holocene at the Sabana de Bogotá region, Northern South America: Archaeological Anthropological Sciences, 10, 817-832. https://doi.org/10.1007/s12520-016-0403-3
Denham, T., Vrydaghs, L., Iriarte, J., 2007, Rethinking agriculture: Archaeological and ethnoarchaeological perspectives: California: Walnut Creek, Left Coast Press, 476p.
Domingo, L., Koch, P.L., Hernández Fernández, M., Fox, D.L., Domingo, S., Alberdi, M.T., 2013, Late Neogene and Early Quaternary paleoenvironmental and paleoclimatic conditions in southwestern Europe: isotopic analyses on mammalian taxa: PLoS One, 8(5), e63739. https://doi.org/10.1371/journal.pone.006373
Ehleringer, J.R., Cerling, T.H., 2002, Stables isotopes, in Mooney, H.A., Canadell, J.G., (eds.), Encyclopedia of global environmental change, Vol. 2. The Earth system: biological and ecological dimensions of global environmental change: Chichester, UK, John Wiley and Sons, 544–550.
Faure, G., 1977, Principles of isotope geology: USA, John Wiley and Sons, 589p.
Gallina, S., Bello, J., Verteramo, C. C., Delfín, C., 2010, Daytime bedsite selection by the texan white-tailed deer in xerophyllous brushland, North-eastern Mexico: Journal of Arid Environments, 74(3), 373-377. https://doi.org/10.1016/j.jaridenv.2009.09.032
Grayson, D.K., 1981, A critical view of the use of archaeological vertebrates in paleoenvironmental reconstruction: Journal of Ethnobiology, 1(1), 28-38.
Guerrero. R., Berlanga, M., 2000, Isótopos estables: fundamento y aplicaciones: Actualidad SEM, Boletín Informativo de la Sociedad Española de Microbiología, 30, 17-23.
Iacumin, P., Bocherens, H., Mariotti, A., Longinelli, A., 1996, Oxygen isotope analyses of co-existing carbonate and phosphate in biogenic apatite: a way to monitor diagenetic alteration of bone phosphate: Earth and Planetary Science Letters, 142(1–2),1–6. https://doi.org/10.1016/0012-821X(96)00093-3
Keeley, J.E., Rundel, P.W., 2003, Evolution of CAM and C4 Carbon-Concentrating Mechanisms: International Journal of Plant Sciences, 164(S3), S55–S77. https://doi.org/10.1086/374192
Koch, P.L., 2007, Isotopic study of the biology of modern and fossil vertebrates, in Michener, R., Lajtha, K. (eds), Stable isotopes in ecology and environmental science, 2nd Ed.: Boston, Blackwell Publishing, 99-154.
Koch, P.L., Tuross, N., Fogel, M.L., 1997, The effects of sample treatment and diagenesis on the isotopic integrity of carbon in biogenic hydroxylapatite: Journal of Archaeological Science, 24(5), 417–429. https://doi.org/10.1006/jasc.1996.0126
Kohn, M.J., 2010, Carbon isotope compositions of terrestrial C3 plants as indicators of (paleo)ecology and (paleo) climate: Proceedings of the National Academy of Sciences, 107 (46), 19691–19695. U.S.A. https://doi.org/10.1073/pnas.1004933107
Lehmann, J., Kleber, M., 2015, The contentious nature of soil organic matter: Nature, 528(7580), 60-68.
Madella, M., Alexandre, A., Ball, T., 2005, International Code for Phytolith Nomenclature 1.0: Annals of Botany, 96, 253-260. https://doi.org/10.1093/aob/mci172
Madella, M., Powers, A., Jones, M., 1998, A simple method of extraction of opal phytoliths from sediments using a non-toxic heavy liquid: Journal Archaeological Science, 25, 801-803. https://doi.org/10.1006/jasc.1997.0226
Marchant, R., Behling, H., Berrío, J., Cleef, A., Duivenvoorden, J., Hooghiemstra, H., Kuhry, P., Melief, R., Schreve, E., Van Geel, B., Van der Hammen, T., Van Reenen, G., Wille, M., 2002, Pollen-based biome reconstructions for Colombia at 3000, 6000, 12000, 15000 and 18000 14C yr ago: Late Quaternary tropical vegetation dynamics: Journal of Quaternary Science, 17(2), 113-129. https://doi.org/10.1002/jqs.669
Martínez-Polanco, M.F., 2016, El Cuy (Cavia sp.), un recurso alimenticio clave en Aguzuque, un sitio arqueológico de la Sabana de Bogotá, Colombia: Latin American Antiquity, 27(4), 521-526. https://doi.org/10.7183/1045-6635.27.4.512
Mora, G., Pratt, L., 2002, Carbon isotopic evidence from paleosols for mixed C3/C4 vegetation in the Bogotá Basin, Colombia: Quaternary Science Reviews, 21 (8), 985-995. https://doi.org/10.1016/S0277-3791(01)00079-8
Pérez, A., 2000, La estructura ecológica principal de la Sabana de Bogotá: Colombia, Sociedad Geográfica de Colombia, 37p.
Piperno, D., 2006, Phytoliths: A comprehensive guide for archaeologists and paleoecologists: Oxford, Altamira Press.
Piperno, D. y Pearsall, D. 1998. The origins of agriculture in the lowland neotropics. San Diego: Academic Press, 304p.
Posada, W., 2017, Arqueología en territorios de incandescencia: una aproximación geográfica a los procesos de cambio social y ambiental bajo condiciones de volcanismo activo. Cordillera central de Colombia: Bogotá, Colombia, Instituto Colombiano de Antropología e Historia, 312p.
Rangel, O., 2000, Colombia diversidad biótica III: la región de vida paramuna de Colombia: Bogotá, Universidad Nacional de Colombia, 852p.
Reizt, E.J., Wing, E.S., 2008, Zooarcheology, 1st ed: New York, Cambrigde University Press, 533p.
Révész, K. M., Landwehr, J. M., 2002, δ13C and δ18O isotopic composition of CaCO3 measured by continuous flow isotope ratio mass spectrometry: statistical evaluation and verification by application to Devils Hole core DH–11 calcite: Rapid Communications in Mass Spectrometry, 16,1012–2114. https://doi.org/10.1002/rcm.833
Rozanski, K., Araguás-Araguás, L., Gonfiantini, R., 1993. Isotopic patterns in modern global precipitation, in Swart, P. K., Lohmann, K. C., Mckenzie, J., Savin, S., (eds.), Climate change in continental isotopic records, Vol. 78, 1-36. https://doi.org/10.1029/gm078p0001
Sánchez, B., 2005, Reconstrucción del ambiente de mamíferos extintos a partir del análisis isotópico de los restos esqueléticos, en Alcorno, P., Redondo, R., Toledo, J., (eds.), Nuevas técnicas aplicadas al estudio de los sistemas ambientales: los isótopos estables: España,Universidad Autónoma de Madrid, 49-64.
Schmidt, M. W., Torn, M. S., Abiven, S., Dittmar, T., Guggenberger, G., Janssens, I.A., Kleber, M., Kögel-Knabner, I., Lehmann, J., Manning, D.A.C., Nannipieri, P., Rasse, D.P., Weiner, S., Trumbore, S.E., 2011, Persistence of soil organic matter as an ecosystem property: Nature, 478(7367), 49-56. https://doi.org/10.1038/nature10386
Smith, B.N., Epstein, S., 1971, Two categories of 13C/12C ratios for higher plants: Plant Physiol, 47(3), 380–384. https://doi.org/10.1104/pp.47.3.380.
Tejada-Lara, J.V., MacFadden, B.J., Bermudez, L., Rojas, G., Salas-Gismondi, R., Flynn, J.J., 2018, Body mass predicts isotope enrichment in herbivorous mammals: Proceedings of the Royal Society Biological Sciences, 285(1881), 20181020. https://doi.org/10.1098/rspb.2018.1020
Tipple, B.J., Meyers, S.R., Pagani, M., 2010, Carbon isotope ratio of Cenozoic CO2: a comparative evaluation of available geochemical proxies: Paleoeceanagraphy and Paleoclimatology, 25(3), PA3202. https://doi.org/10.1029/2009PA001851
Triana, A.V., 2019, Dieta y acceso a recursos determinadas a partir del sexo en grupos de cazadores recolectores de la sabana de Bogotá durante el Holoceno temprano y medio: Bogotá, Colombia, Universidad de los Andes, P.hD. Dissertation.
Triana, A.V., Sedov-Sedov, S., Salinas-Acero, J., Carvajal-Contreras, D., Moreano, C., Tovar-Reyes, M., Solleiro-Rebolledo, E., Díaz-Ortega, J., 2019, Environmental reconstruction spanning the transition from hunter/gatherers to early farmers in Colombia: paleopedological and archaeological indicators from the pre-ceramic sites Tequendama and Aguazuque: Quaternary International, 516, 175-189. https://doi.org/10.1016/j.quaint.2018.09.048
Triana, A.V., Morales, P., Casar, I., Salinas, J., 2020a, Análisis de Isótopos estables en restos óseos humanos y de fauna en los sitios arqueológicos del Holoceno temprano y medio Tequendama y Aguazuque (Sabana de Bogotá, Colombia): Jangwa Pana 19(1), 10-22. https://doi.org/10.21676/16574923.3432
Triana, A.V., Vélez-Bedoya, S., Sedov, S., Solleiro-Rebolledo, E., Díaz-Ortega, J., 2020b, Quantitative analysis of micromorphological images in edaphosedimentary sequences of archaeological sites of Tequendama and Aguazuque, Sabana de Bogotá, Colombia: Boletín Geológico, Servicio Geológico Colombiano, 47, 107-122. https://doi.org/10.32685/0120-1425/boletingeo.47.2020.495
Twiss, P., Suess, E., Smith, R., 1969, Morphological classification of grass phytoliths: Soil Science Society of America Proceedings, 33 (1), 109-115. https://doi.org/10.2136/sssaj1969.03615995003300010030x
Van der Hammen, T., Urrego, G., Klinken, G., 1990, Isótopos estables y dieta del hombre prehistórico en la sabana de Bogotá (un estudio inicial): Boletín Arqueología-FIAN, 5 (2), 3-10.
Van der Hammen, T., 1992, Historia, ecología y vegetación: Bogotá, Corporación Colombiana para la Amazonia, 411p.
Weiner, S., 2010, Microarchaeology. Beyond the visible archaeological record, 1st ed.: New York, Cambridge University Pres, 414p.
Werner, R.A., Brand, W.A., 2001, Referencing strategies and techniques in stable isotope ratio analysis: Rapid Communications in Mass Spectrometry, 15(7), 501–519. https://doi.org/10.1002/ rcm.258
Wynn, J. G., Harden, J. W., Fries, T. L., 2006, Stable carbon isotope depth profiles and soil organic carbon dynamics in the lower Mississippi Basin: Geoderma, 131(1-2), 89-109. https://doi.org/10.1016/j.geoderma.2005.03.005
Peer Reviewing under the responsibility of Universidad Nacional Autónoma de México.
This is an open access article under the CC BY-NC-SA license(https://creativecommons.org/licenses/by-nc-sa/4.0/)

